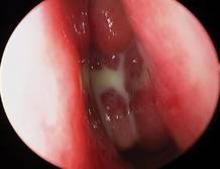
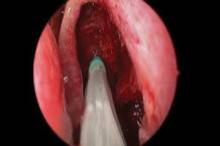
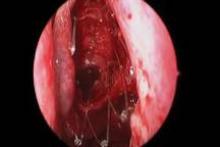
CHICAGO – A bioabsorbable steroid-eluting stent preserved sinus patency following endoscopic sinus surgery in 50 patients with chronic rhinosinusitis in a prospective, multicenter study.
The stent was associated with low rates of polyp formation, inflammation, and adhesions, despite a challenging patient population and withholding of postoperative oral and topical steroids for 30 days, lead author Dr. Keith D. Forwith said at the Combined Otolaryngology Spring Meetings.
The results were consistent across different patient populations and consistent with the pilot study in 43 patients (Int. Forum Allergy Rhinol. 2011;1:23-32).
"I think with any kind of new technique or device, the ultimate thing is how the surgeon feels about it and his or her results," he said. "What I noticed most of all when we stopped doing the study is that I missed using this device. Patients I had used it in during the study had very clean results, very easy debridements. ... We’re very pleased with the results and think it’s a promising technology."
Study sponsor Intersect ENT Inc. has submitted the investigational stent for federal review and hopes to market the device within 6-9 months.
The springlike stent maintains patency by propping open the sinus cavity and releasing mometasone furoate into the sinus lining over 30 days before being resorbed. If approved, it could provide patients with an alternative to space-filling packing materials and silicone stents, and reduce the use of systemic steroids.
The current study involved 50 adults from seven centers with chronic rhinosinusitis for at least 8 consecutive weeks that was confirmed by CT scan (mean CT stage, 11.2). Antibiotics could be prescribed per physician standard of care, and saline irrigation was permitted postoperatively as needed. Polyps were present in 66% of patients, and 28% had undergone a prior sinus procedure. Their mean age was 44 years, and 52% were male.
Ethmoidectomy and maxillary antrostomy were performed in all 50 patients, with 28 also undergoing frontal sinusotomy and 31 sphenoidotomy. Stents were placed unilaterally in 10 patients and bilaterally in 40 patients. Device placement was successful in 100% of sinuses treated, and the implants were resorbed as predicted, Dr. Forwith said. At postoperative day 30, 15% of the material remained and 0.2% remained at day 60.
Endoscopic follow-up at 1 month revealed polypoid edema in 10% of patients, significant adhesion formation in 1%, and middle turbinate lateralization in only 4.4%, said Dr. Forwith, who is in private practice in Louisville, Ky.
When patients were asked about the procedure, their mean score on the 22-question Sino-Nasal Outcome Test improved significantly from baseline through 6 months. The same was true using the Rhinosinusitis Disability Index. "We were pretty pleased that our patients liked the results of the intervention," he said.
No clinically significant changes from baseline occurred in lens opacities or intraocular pressure, which was a theoretical concern given the close proximity of the device. The patients’ mean intraocular pressure was 15 mm Hg at baseline and 14.3 mm Hg at day 30.
One patient experienced headache with sinus pressure/irritation at day 21 that was determined to be related primarily to the surgery and was exacerbated in intensity by the presence of crust on the device. The device was removed and the event resolved without sequelae by day 28.
When asked during a discussion of the study whether the drug-eluting stent resulted in any systemic complications or adrenal corticol suppression, Dr. Forwith replied that they did not specifically look at adrenal suppression, but added that the 370-mcg dose of mometasone furoate is lower than the dose patients receive with most b.i.d. nasal spray administration.
Stent maker Intersect ENT provided funding, administrative support, and materials for the study. Dr. Forwith reported no conflicts of interest.