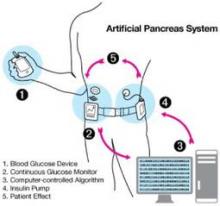
The U.S. Food and Drug Administration has issued draft guidance to assist investigators and manufacturers in the development of artificial pancreas device systems to treat type 1 diabetes.
The 64-page document, issued Dec. 6, provides recommendations for design and testing that meet statutory requirements for safety and effectiveness for artificial pancreas devices, several of which are currently under development. These automated closed-loop systems combine a continuous glucose monitor (CGM), an insulin infusion pump, and a glucose meter for calibrating the monitor. The devices would work together, monitoring blood glucose levels and automatically infusing appropriate doses of insulin as determined by a computer algorithm.
"While not a cure, an artificial pancreas could reduce dangerous high and low blood sugars, providing a better quality of life for those with diabetes and lowering the risk for future diabetes-related complications," said Charles "Chip" L. Zimliki, Ph.D., leader of the FDA’s Artificial Pancreas Working Group and Critical Path Initiative, in a media telebriefing.
One of the primary goals of diabetes researchers and of the diabetes community has been to develop "an automated system that could replace the endocrine portion of the pancreas to control blood glucose levels. People with type 1 diabetes must frequently monitor their blood sugar using a blood glucose meter throughout the day, and adjust their insulin dosing based upon these readings. It’s difficult, and we need devices to make it easier," Dr. Zimliki said.
The guidance addresses different types of future generation artificial pancreas devices, such as a treat-to-range system that would adjust insulin dosing if glucose levels approach a low or high threshold, and a fully automated treat-to-target system that would maintain target glucose levels at all times, without interaction from the user other than CGM calibration.
A three-phase clinical study progression is recommended to facilitate moving trials to outpatient settings as quickly as possible. Sponsors are permitted to use existing safety and effectiveness data for artificial pancreas device components, including data gathered from clinical studies conducted outside of the United States. They are also given the choice of either showing that the system provides glycemic control as well as standard therapies or that it provides better glycemic control when compared with other therapies, Dr. Zimliki said.
He noted that flexibility is a key feature of the artificial pancreas guidance, which is one of the few from FDA to be issued prior to the approval of an actual device. The document allows flexibility with regard to study size and duration, end points, and methodologies. "Because of the novelty of these systems, we really couldn’t be prescriptive in this guidance document. ... We have recommendations in there, and if people want to follow them, that’s great. But if they don’t, they just have to provide us justification for what they want to do."
Thus far, the FDA has approved 20 clinical trials evaluating various artificial pancreas device systems, and "the data are very encouraging," he said.
Regarding a possible timeline for device approval, Dr. Zimliki said that FDA’s main responsibility is to "develop a clear pathway forward" for investigators and manufacturers, and that "These systems aren’t going to be perfect right out of the gate, and there’s going to be an iterative step approach. ... We really don’t know the actual timeline, but we encourage this to happen as fast as possible."
The current guidance was informed by public comments on a previous one issued last June outlining FDA’s study expectations for a low-glucose suspend (LGS) device component that would respond to sensor readings of low or rapidly declining blood glucose levels by temporarily reducing or shutting down the delivery of insulin in order to avoid or mitigate hypoglycemia. The FDA "will be looking forward to" comments on the current document as well, Dr. Zimliki said. It will be published in the Federal Register in a few days.