The compounding pharmacy at the heart of the ongoing federal and Massachusetts state investigation into contaminated lots of methylprednisolone acetate has been accused of illegally selling the medication in large batches across state lines, a development that prompted officials during an Oct. 11 telebriefing on the meningitis outbreak to call for improved oversight of such pharmacies at the federal level.
The actions of the New England Compounding Center (NECC) in Framingham, Mass., the pharmacy that has been the focus of the investigation into contaminated preparations of the steroid, would constitute it to be a drug manufacturer under Massachusetts law, which allows compounding only on the receipt of a patient-specific prescription. The NECC had sold the contaminated lots to clinics in 23 states before the Food and Drug Administration worked with the pharmacy to recall the steroid on Sept. 26, and then on Oct. 6 voluntarily recalled all of the compounded products in circulation that had been made at its Framingham, Mass., facility. The company voluntarily shut down its operations Oct. 3.
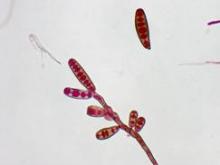
Fungal meningitis cases and deaths in people who received epidural injections of the contaminated methylprednisolone acetate continue to rise, but investigators at the FDA and the Centers for Disease Control and Prevention have begun to determine which fungal species are present in patients and in contaminated vials of the steroid. Many tests are still unfinished.
Cultures of cerebrospinal fluid and histopathologic analysis of specimens have so far indicated fungal infection with Aspergillus species in 1 patient and Exserohilum species in 10 patients. The FDA has found fungal contamination by direct microscopic examination of foreign matter taken from sealed vials of the steroid collected from the NECC and from clinics that received the recalled lots.
"We know that fungus such as Exserohilum can be difficult to detect in samples from patients. Patients and their physicians should not assume fungal testing that is negative means there is no infection," Dr. J. Todd Weber, the CDC’s incident manager of the multistate meningitis outbreak, said during the telebriefing. In these cases, patients should still be treated for fungal meningitis. He noted that Exserohilum previously has not been observed to cause fungal meningitis, which historically is itself "very rare."
Dr. Weber reported that of the nearly 14,000 people who received an epidural or intra-articular injection with the steroid from recalled lots, more than 12,000 have now been contacted and those with symptoms of meningitis or possible joint infection have been referred to their physicians.
As of Oct. 11, the outbreak has caused 169 cases of meningitis and 14 deaths in 11 states: Florida (7 cases, including 2 deaths), Indiana (21 cases, including 1 death), Tennessee (49 cases, including 6 deaths), Maryland (13 cases, including 1 death), Michigan, (38 cases, including 3 deaths), North Carolina (2 cases), Virginia (30 cases, including 1 death), Ohio (3 cases), New Jersey (2 cases), Minnesota (3 cases), and Idaho (1 case). The meningitis cases have all been linked to epidural injections with methylprednisolone acetate compounded by the NECC. Production lots of the steroid at doses of 40 mg/mL and 80 mg/mL have been recalled, as well as all other products currently in circulation that were compounded at and distributed from the facility in Framingham.
The CDC is currently investigating one case of an ankle joint infection associated with an injection of the steroid in Michigan, but the laboratory results have not been completed to confirm whether or not it was caused by fungal infection, according to Dr. Weber. "We expect that through additional patient notification efforts, we may see additional patients come forward with infections of the joints. These patients may present with symptoms, including fever [and] increased pain, redness, warmth, or swelling in the joint where they received the injection or at the injection site."
In addition to identifying and assisting patients who may have been potentially exposed to the contaminated drug, the Massachusetts Department of Pharmacy and the FDA are reviewing the processes and procedures of the NECC. The FDA is also looking into the source of the ingredients used to make the compounded methylprednisolone acetate, said Deborah M. Autor, J.D., deputy commissioner for global regulatory operations and policy at the FDA.
FDA investigations during Oct. 2-10 ruled out Busse Hospital Disposables Inc. in New York as a possible source of at least one case at a Tennessee pain clinic as well as epidural trays from a New Orleans pain clinic that had been central to the outbreak, Dr. Autor said.