SILVER SPRING, MD. – Bedaquiline, an oral antimycobacterial drug with a novel mechanism of action, received an 18 to 0 vote for approval for the treatment of multidrug-resistant pulmonary tuberculosis by a Food and Drug Administration advisory panel.
At a meeting on Nov. 28, the FDA’s Anti-Infective Drugs Advisory Committee concluded that phase II clinical data indicated the drug increased the time to sputum culture conversion, a surrogate marker for clinical effectiveness, when added to standard background treatment in adults with multidrug-resistant (MDR) TB. Sputum culture conversion was defined as two consecutive negative cultures collected at least 25 days apart that were not followed by a confirmed positive culture.
The panel voted 11 to 7 that the data provided substantial evidence that the drug was safe for this indication. There were 10 deaths among 79 patients on bedaquiline, compared with 2 deaths in 81 patients on placebo. The FDA reviewers and the company could not identify any pattern or cause that could explain the imbalance in the death rate. All but one of the deaths in the bedaquiline-treated patients occurred after treatment with the drug was completed.
The FDA is reviewing the drug as an accelerated approval, because bedaquiline addresses the unmet need for an effective therapy for MDR TB. In such cases, recommendation for approval can be based on surrogate clinical end points, with the requirement that clinical effectiveness be confirmed in a postmarketing study with hard clinical end points before full approval is granted. Panelists recommended that full approval require concrete evidence of increased cure rates with treatment in a confirmatory study. Further, more data on the drug are needed in HIV-positive and black populations, and studies also are needed to address the implications of the drug’s long half-life.
Bedaquiline, a diarylquinolone discovered at Janssen Therapeutics, a division of Janssen Products, inhibits mycobacterial adenosine triphosphate (ATP) synthetase. The drug kills both replicating and nonreplicating TB bacilli, and is active against drug-sensitive and MDR TB, according to the company.
If approved by the FDA, bedaquiline will be the first antituberculosis drug with a novel mechanism of action approved in the United States since rifampin’s approval in 1970, and the first drug approved for TB since rifapentine was approved in 1998.
"The fact that this drug is able to shorten the period the organism is in the sputum has public health implications for [reducing]transmission," said Dr. Peter Katona, of the University of California, Los Angeles.
In 2011, 98 cases of MDR TB were reported in the United States, but the condition is a far greater problem globally, with an estimated incidence in 2011 of 310,000 cases, according to the Centers for Disease Control and Prevention.
The panel reviewed two phase II studies of almost 400 patients with pulmonary MDR TB.
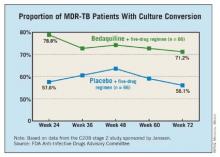
One study enrolled 160 nonwhite patients, most of them men from South Africa with a mean age of 35 years. All were newly diagnosed with pulmonary MDR TB; a small proportion were HIV positive. They were treated with a standard five-drug regimen that included ethionamide, pyrazinamide, ofloxacin, kanamycin, and 400 mg once daily of bedaquiline for 2 weeks, followed by 200 mg three times a week for 22 weeks, or placebo. After 24 weeks, patients continued background treatment for 12-18 months.
At 24 weeks, 79% of those treated with bedaquiline had a culture conversion, compared with 58% of those on placebo, a significant difference.
The second study was an open-label trial of 233 previously treated patients with sputum smear–positive pulmonary MDR TB. They received the same dosing regimen of bedaquiline combined with an individualized background regimen for MDR TB. At 24 weeks, the culture conversion rate was 80%, and sputum cultures became negative in a mean of 57 days. The faster conversion rate likely reflected the fact that most patients were already on treatment when enrolled in the trial.
The incidence of serious adverse events was higher among those on bedaquiline (6.9% vs. 1.9%). Treatment was associated with elevated transaminases in 4 cases, compared with 0 in placebo-treated patients. There was a modest increase in QT prolongation, although there were no cases of torsades de pointes or evidence that this caused any deaths.
The FDA’s deadline for making a decision on bedaquiline is by the end of December. The agency usually follows the recommendations of its advisory panels, which are not binding. Panelists were cleared of potential conflicts of interest related to the topic of the meeting.