Safety
Valdecoxib and placebo had comparable upper gastrointestinal tract ulceration rates, whereas naproxen produced a significantly higher incidence of upper gastrointestinal tract ulcers than did 5 and 10 mg valdecoxib and placebo (P < .05). There were 14 adjudicated symptomatic ulcers during the study: 1 in the 5-mg valdecoxib group, 2 in the 10-mg valdecoxib group, 3 in the 20-mg valdecoxib group, and 7 in the 500-mg naproxen group.
Adverse events with an incidence of at least 5% in any treatment group and adverse events leading to withdrawal from the study are summarized by body system in Table 4. There were no significant differences in the incidence of adverse events between the valdecoxib and placebo groups. In contrast, 500 mg naproxen twice daily was associated with significantly more adverse events than 5 or 10 mg/day valdecoxib (P < .05). The incidence of adverse events was similar in the 20-mg valdecoxib and naproxen groups. Most adverse events were reported in the gastrointestinal system and consisted of abdominal pain, constipation, diarrhea, dyspepsia, flatulence, and nausea. The incidences of constipation, diarrhea, and flatulence were significantly higher in the naproxen group than in the 5-, 10-, and 20-mg valdecoxib groups, respectively. Other adverse events included accidental injury, headache, myalgia, and upper respiratory tract infections. Valdecoxib at 5 mg/day produced a significantly higher incidence of myalgia than did placebo, and valdecoxib at 20 mg/day produced a significantly lower incidence of upper respiratory tract infections than did placebo. Adverse events causing withdrawal with an incidence of at least 1% were accidental injury, abdominal pain, diarrhea, dyspepsia, nausea, abnormal hepatic function, rash, and blurred vision. The proportion of patients in the naproxen group (12.7%) who withdrew from the study was significantly greater than those for the 5-and 20-mg valdecoxib (6.0% and 5.5%) groups (P < .05), although the incidence of withdrawal due to adverse events in the 10-mg valdecoxib and naproxen groups were similar. In addition, gastrointestinal adverse events commonly related to NSAID treatment, such as dyspepsia and constipation, were more frequent in the naproxen group than in the valdecoxib and placebo groups.
TABLE 4
Adverse events
| Valdecoxib | Naproxen | ||||
|---|---|---|---|---|---|
| Placebo (n = 178) | 5 mg qd (n = 188) | 10 mg qd (n = 174) | 20 mg qd (n = 185) | 500 mg bid (n = 183) | |
| Incidence ≥ 5% in any treatment group | |||||
| Total | 109 (53.2) | 112 (55.7)† | 113 (55.1)† | 121 (60.2) | 139 (68.1)* |
| Accidental injury | 11 (5.4) | 3 (1.5) | 10 (4.9) | 12 (6.0) | 9 (4.4) |
| Headache | 11 (5.4) | 12 (6.0) | 7 (3.4) | 14 (7.0) | 9 (4.4) |
| Abdominal pain | 19 (9.3) | 14 (7.0) | 18 (8.8) | 13 (6.5) | 25 (12.3) |
| Constipation | 6 (2.9) | 4 (2.0) | 1 (0.5)† | 4 (2.0) | 12 (5.9) |
| Diarrhea | 10 (4.9) | 7 (3.5) | 14 (6.8) | 11 (5.5) | 12 (5.9) |
| Dyspepsia | 15 (7.3) | 22 (10.9) | 22 (10.7) | 20 (9.9)† | 35 (17.2)* |
| Flatulence | 12 (5.9) | 7 (3.5) | 5 (2.4)† | 9 (4.5) | 14 (6.9) |
| Nausea | 10 (4.9) | 18 (9.0) | 17 (8.3) | 9 (4.5) | 10 (4.9) |
| Myalgia | 0 (0.0) | 13 (6.5)*† | 3 (1.5) | 2 (1.0) | 1 (0.5) |
| Upper respiratory tract infections | 18 (8.8) | 9 (4.5) | 10 (4.9) | 7 (3.5)* | 10 (4.9) |
| Incidence ≥ 1% in any treatment group causing withdrawal | |||||
| Total | 17 (8.3) | 12 (6.0)† | 18 (8.8) | 11 (5.5)† | 26 (12.7) |
| Accidental injury | 2 (1.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| Abdominal pain | 5 (2.4) | 2 (1.0) | 6 (2.9) | 2 (1.0) | 7 (3.4) |
| Diarrhea | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) | 3 (1.5) |
| Dyspepsia | 2 (1.0) | 2 (1.0) | 3 (1.5) | 1 (0.5)† | 9 (4.4)* |
| Nausea | 2 (1.0) | 1 (0.5) | 2 (1.0) | 1 (0.5) | 2 (1.0) |
| Abnormal hepatic function | 0 (0.0) | 2 (1.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Rash | 0 (0.0) | 2 (1.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Blurred vision | 2 (1.0) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| *P < .05 vs placebo. | |||||
| † P < .05 vs naproxen. | |||||
| ‡ Data are presented as number (%) of patients reporting events. | |||||
| bid, twice daily; qd, once daily. | |||||
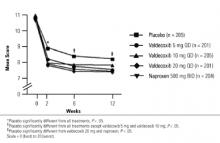
FIGURE 2
Western Ontario and McMaster’s Universities Osteoarthritis Pain Index
Discussion
This study confirmed that the novel COX-2–specific inhibitor valdecoxib at a dosage of 10 or 20 mg/day is as effective as naproxen at a dosage of 500 mg twice daily in relieving moderate to severe osteoarthritis of the knee over 12 weeks. In addition, treatment with 10 mg/day valdecoxib orally, the recommended dosage for treatment of osteoarthritis, is associated with a significantly lower gastroduodenal ulceration rate than occurs with the conventional NSAID, naproxen.
Patients receiving 10 and 20 mg/day valdecoxib experienced significant improvements in the signs and symptoms of osteoarthritis, and in all assessments the efficacies of valdecoxib 10 and 20 mg/day were numerically similar to that of naproxen. This finding is consistent with the inhibition of prostaglandin production in inflamed synovial tissue and in the central pain pathway. Increased COX-2 activity in the spinal cord in response to tissue damage and in the synovial membrane of osteoarthritis patients is at least partly responsible for joint inflammation and sensitization to inflammatory pain.14–16 The efficacy of valdecoxib in treating moderate to severe osteoarthritis of the knee was consistent with reports of other COX-2–specific inhibitors that are comparable to conventional NSAIDs in relieving chronic pain and inflammation.17,18 These data confirmed that 10 mg/day valdecoxib is as effective as 500 mg naproxen twice daily in treating the pain and inflammation associated with osteoarthritis. The efficacy of 10 mg/day valdecoxib makes it one of the most potent COX-2–specific inhibitors for treating moderate to severe osteoarthritis.