- Consider injections of hyaluronic acid only after conservative therapy has been tried for at least 3 months or the patient is unable to tolerate NSAIDs.
- Stress to patients that pain relief may not be fully experienced until 5 to 7 weeks following the last injection.
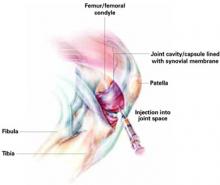
Hyaluronic acid injections can help relieve pain for carefully selected patients with knee osteoarthritis. But this option should be reserved for those whose pain has not responded to adequate trials of systemic therapeutic agents (acetaminophen, nonsteroidal anti-inflammatory drugs [NSAIDs], cyclooxygenase-2 [COX-2] inhibitors), topical agents, or to lifestyle modifications such as weight reduction and exercise.
Hyaluronic acid injections may also be indicated when knee surgery must be delayed for middle-aged persons.1
In spite of the Food and Drug Administration’s approval of this therapy, uncertainty about its efficacy exists among the medical community. A recent meta-analysis of the effectiveness of intra-articular hyaluronic acid for knee osteoarthritis that included 22 published and unpublished, English and non-English, single or double-blinded, randomized controlled trials in humans showed that hyaluronic acid has only a small effect on pain relief when compared with placebo.2
We provide here a stringent test of the efficacy of viscosupplementation for relieving knee pain from osteoarthritis with a meta-analysis that includes only data from randomized, double-blinded, controlled trials of hyaluronic acid that measured pain using a visual analogue scale (VAS), the most widely accepted method for pain evaluation.
Long time coming
Balazs first proposed hyaluronic acid as a treatment for patients with arthritic diseases in 1942. In the early 1970s, therapeutic studies were begun to test the efficacy of hyaluronic acid on knee osteoarthritis. The results were encouraging and side effects were few.28 With the FDA’s approval in 1998, intra-articular “viscosupplementation” with hyaluronic acid—also called hyaluronan or hyaluronate, and the hylan derivatives of hyaluronic acid—is a welcome option for many of the 16 million older Americans with osteoarthritis of the knee.1
Methods
Selection of studies
We identified clinical trials of viscosupplementation with hyaluronic acid in humans published in English from 1965 through August 2004 through a computerized literature search of Medline. The keyword used was “hyaluronic acid,” which was combined with “trial” or “osteoarthritis knee” or “viscosupplementation.” We conducted an additional manual search of the reference lists of included articles and review articles. We also searched the Cochrane Library and websites of the Agency for Healthcare Research and Quality (AHRQ) for information on hyaluronic acid in knee osteoarthritis. We identified 1872 articles with this search process.
Of the 1872 articles, we identified by title and abstract 33 that might be pertinent to this study, including 17 randomized trials. We excluded reviews, meta-analyses, comparison trials, and trials reporting VAS as part of the WOMAC (Western Ontario McMaster Universities Index) scale. We attempted to contact authors of the studies that used a double-blind, randomized controlled design, to obtain any data that may not have been included in the publications. Three authors provided the details requested; the others did not respond or stated that additional data were not available. Of the 17 randomized trials we identified, 8 were excluded because they were open, single-blinded, or did not use the VAS to measure pain outcomes.3-10 The remaining 9 double-blinded, placebo controlled, randomized clinical trials of viscosupplementation with hyaluronic acid for knee osteoarthritis that did use a VAS to measure pain were included in this meta-analysis (TABLE 1). Because one of the studies (by Henderson) included 2 subgroups of pain severity, these were considered as 2 separate trials. The trial by Petrella had 2 treatment groups, one with only hyaluronic acid and the other with hyaluronic acid and NSAIDs. We considered them separately in the analysis, resulting in a total of 11 clinical trials for the meta-analysis.19 Henceforth in this report, we will refer to 11 rather than 9 clinical trials.
Extraction of data
Two investigators independently extracted the following data for each study: year of publication, study design, mean age, number of patients enrolled in each treatment group, number of doses of treatment used, and outcomes measured. When disagreements between investigators occurred, the point of disagreement was discussed until a consensus was reached. Since the treatment duration and the time post-treatment when pain was assessed varied among the trials, we grouped outcomes into four time intervals: at 1 week, 5 to 7 weeks, 8 to 12 weeks, and 15 to 22 weeks after the last hyaluronic acid injection.