The year 2007 was rather calm, compared to the 3 previous years in regards to new vaccines and vaccine recommendations. Although no breakthrough vaccine products came onto the market in 2007, there were new recommendations and licensure for new age groups for existing vaccines and a recall of some lots of Hib vaccines.
Meningococcal vaccine
Recommendations on the use of the quadrivalent meningococcal conjugate vaccine (MCV4) have evolved since its licensure in 2005 for use in persons 11 to 55 years of age. The first set of recommendations focused on universal vaccination of preteens, aged 11 to 12, those entering high school who had not received the vaccine previously, and others at risk for meningococcal disease including college freshmen living in dormitories.1 The MCV4 was preferred to the older polysaccharide vaccine (MPSV4) which was recommended only for children aged 2 to 10 and adults over age 55 at increased risk.
In 2007, the CDC changed 2 of the 2005 recommendations:
- The first, in August, simplified the recommendations for teens, making MCV4 universally recommended for all those aged 11 to 18 at the earliest opportunity.2
- The second, in December, followed FDA approval for use of MCV4 in children aged 2 to 10 years. The CDC now recommends MCV4 as the preferred vaccine in this age group for those at risk (TABLE 1).3
TABLE 1
Populations at increased risk for meningococcal disease who should receive quadrivalent meningococcal conjugate vaccine
|
If someone at ongoing risk for meningococcal disease has been previously vaccinated with MPSV4, they should be revaccinated 3 years later with MCV4. It is not known if repeat doses of MCV4 will be needed, and if so, after what amount of time.
The MCV4 has been linked to Guillain-Barré syndrome (GBS), and a history of GBS is a precaution for its use. For those with a history of GBS who need protection against meningococcal infection, MPSV4 is an alternative.
Hepatitis A vaccine
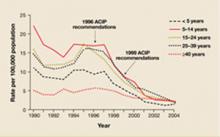
Widespread use of inactivated hepatitis A vaccine (HAV), first licensed in 1995, has markedly reduced the incidence of hepatitis A infection (FIGURE). Recommendations for its use have been periodically revised; current recommendations include universal vaccination of all children at age 12 to 23 months, catch-up vaccination in older children in areas of high prevalence, and vaccination of those at increased risk for hepatitis A including travelers to endemic areas, users of illicit drugs and men who have sex with men.4
FIGURE
Reduction in incidence of hepatitis A infection
Source: Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2006; 55(RR-07).
For those unvaccinated who are acutely exposed to hepatitis A virus and those traveling to areas of high prevalence who do not have time to complete the 2 doses of HAV, the only prevention available until recently has been IG. This has now changed and HAV can be used in both groups. The new recommendation for postexposure prophylaxis is that either a single dose of HAV or use of IG is acceptable.5 At ages 12 months to 40 years, vaccine is preferred. For those over age 40, IG is preferred but vaccine is acceptable. For children less than 12 months, the immune suppressed, and those with chronic liver disease, IG should be used.
Those traveling or working in countries with high rates of hepatitis A can be protected with either HAV or IG. A single dose of HAV is sufficient for healthy people, with a second dose at the recommended interval to complete the series. Those under age 12 months, those who choose not to receive the vaccine, and those who are allergic to the vaccine should be offered IG. Both IG and HAV should be considered for individuals who plan to travel within 2 weeks of the first HAV dose; those over age 40, the immune compromised, and those with chronic liver disease or other chronic medical conditions.
Live attenuated influenza vaccine
FluMist, the live attenuated influenza vaccine (LAIV), which is administered as an intranasal spray, is now approved for use among those 2 to 4 years of age.6 Previously, the LAIV was approved only for healthy, non pregnant persons, 5 to 49 years of age. The LAIV may actually be the preferred product in children as it has been shown to prevent more influenza illness than the trivalent inactivated vaccine (TIV). The LAIV should not be used in anyone with a condition listed in TABLE 2 and should not be administered to children under age 5 who have recurrent wheezing.