• Consider using corticosteroid injections to inhibit collagen synthesis and stimulate enzymatic degradation of existing keloid collagen. B
• Turn to treatment combinations for refractory keloids; regimens may include corticosteroid injections, surgical excision, pressure, occlusive dressings, or radiation. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Keloids are an ongoing clinical challenge. Despite the availability of multiple treatments, choosing an effective therapeutic regimen for these fibroproliferative scars can be vexing, as keloids usually recur.
This review provides a guide to determining which regimen is best for your patient. But first, a word about the causes of these claw-like growths and which patients are at highest risk of developing them.
Even superficial injuries can cause keloids
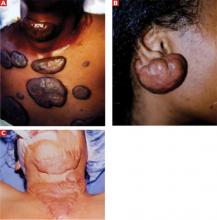
Keloids arise after a disruption of skin integrity following superficial or deep injuries (FIGURES 1A-C). Causes include physical trauma such as cuts, scratches, and insect bites; iatrogenic trauma as in vaccinations or surgical procedures; thermal or chemical burns; and skin eruptions such as chicken pox.
FIGURE 1
Keloids induced by scratches (A), ear piercing (B), and thermal burns (C)
Common sites of keloid development include the ears, jaw, neck, clavicle and sternum, and shoulders. Less commonly, keloids occur on the back, abdomen, and extremities. Rarely, keloids develop on the palms and soles, face, or mucous membranes.1
A minor keloid is smaller than 0.5 cm, while a major keloid is larger. No upper growth limit has been identified, as keloids seem to enlarge indefinitely.2 Keloid scars have a firm and inflexible texture, a shiny appearance, and are elevated above skin level. They are usually flesh-colored, but may be erythematous or hyperpigmented.1 (A similar aberrant wound healing condition often confused with keloids is hypertrophic scarring. See “Differentiating keloids from hypertrophic scars”).
Distinguishing between keloids and hypertrophic scars can be challenging, because both types of scars arise histologically from excessive collagen buildup during the wound healing process. However, there are 3 distinctive characteristics of keloids that hypertrophic scars lack:
- Keloids enlarge beyond wound margins, often with irregular shapes, while hypertrophic scars remain within the confines of the original wound and tend to be linear along scars.
- Keloids do not regress on their own; hypertrophic scars tend to regress spontaneously within a few years.
- Keloids may take months to years to develop, while hypertrophic scars usually appear within 4 to 6 weeks of a traumatic incident.
Source: Gauglitz G, Korting H, Pavicic T, et al. Hypertrophic scarring and keloids: pathomechanisms and current and emerging treatment strategies. Mol Med. 2011;17:113-125.
Although keloids have an aggressive growth pattern, they are considered benign tumors, because the development of malignant cells in a keloid is rare.1 Most patients seek medical attention for cosmetic concerns. However, tissue bulk can cause functional problems, and some keloids are symptomatic with pruritus (27%) or pain (19%).1,3
Who is affected?
The most consistent risk factor for keloid development is a previous keloid. Individuals with deeply pigmented skin appear to be at higher risk, with an estimated prevalence of 4% to 16% among individuals of African, Asian, and Hispanic ancestry.3 Although the rate of keloid occurrence among Caucasians has not been established, keloids have been reported in individuals of nearly every skin color, except albinos.3 Keloids occur in both sexes with similar frequency, and are most likely to develop during puberty and early adulthood—between the ages of 10 and 30 years—but can occur at any age.1,3
Although no clear familial inheritance pattern has been identified, keloids are associated with blood type A and human leukocyte antigens (HLA)-B14, -B21, -BW35, -DR5, -DRB1, -DQA1, -DQB1, and -DQW3.3,4 Emerging research indicates that mutations of the CDC2L1 gene—which encodes a protein kinase essential to cell cycle control—correlate with keloid formation.4
Wound healing gone awry
Normal wound healing involves an overlapping series of processes, including hemostasis, inflammation, and granulation and remodeling. Various mechanisms have been identified for keloid pathology, although no single definitive trigger is known.
Hemostasis. In normal hemostasis, platelets aggregate around a fibrin clot in a wound, stimulating the release of growth factors,3 which precipitate the migration of fibroblasts to the granulation-tissue scaffolding for wound healing. Keloids have increased fibroblast proliferation, with up to 20-fold increased production of Type 1 collagen.1
Inflammation. During the inflammation phase, cytokine cascades stimulate cell proliferation from inflammatory, endothelial, epithelial, and fibroblast cell lines. Keloids have altered levels of many cytokines, resulting in increased inflammatory activity, such as histamine release by mast cells.3
Granulation and remodeling. During normal granulation and remodeling, fibroblasts aggregate and produce extracellular matrix components. The keloid fibroblasts produce higher levels of collagen, elastin, fibronectin, and proteoglycans, but lower levels of hyaluronic acid.3,5 Keloid fibroblasts also make lower levels of tissue plasminogen activator inhibitor (TPA-I), with inferior breakdown of scaffold structures and collagens. While collagen bundles run parallel to the epithelial surface in normal skin, keloid collagen fibers are larger, thicker, and randomly oriented in dense sheets, swirls, or nodules.1