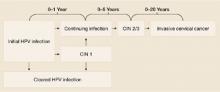
Infection cannot always be cleared. Most HPV infections (whether high-risk or low-risk type) are asymptomatic and are efficiently cleared (ie, no detection of DNA for a specific HPV type) by the immune system.21,22 However, if the infection cannot be cleared or controlled by the immune system, it may become a persistent infection.
Persistent infection with HPV increases the probability of progression to high-grade cervical intraepithelial neoplasia (CIN) and invasive carcinoma ( FIGURE ).18-19 Evidence also increasingly shows that high-risk HPV types likely cause anal, penile, scrotal, vulvar, vaginal, and some head and neck cancers.25
After HPV vaccination, neutralizing antibodies are secreted from memory B cells, and bind to their target HPV type, preventing infection before it occurs, thereby blocking the initial step toward development of cervical cancer.
15 high-risk oncogenic types. Papillomaviruses such as HPV are nonenveloped, double-stranded, DNA viruses. They infect cutaneous and mucosal epithelial tissues. More than 100 HPV types have been identified,3 about 30 to 40 of which are spread by sexual contact.4 Of the many known HPVs, only 15 are high-risk oncogenic types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68, and 73) that can cause cervical cancer.5.6 Of these high-risk oncogenic types, HPV 16 and 18 account for about 70% of all cervical cancers.7
The new HPV vaccines, Gardasil and Cervarix, (see Web table) both contain virosomal antigens to vaccinate against HPV types 16 and 18. Persistent infection with these high-risk HPV types is necessary for the development of cervical cancer. Chronic infection with low-risk HPV types (eg, HPV 6 or 11) may lead to the development of anogenital warts and other low-grade genital abnormalities, as well as laryngeal cancer or recurrent respiratory papillomatosis. Gardasil also contains virosome antigens for these 2 HPV types. Warts on the hands are usually attributable to HPV 7.8
Viral integration is a necessary step in the malignant transformation of HPV infection; infection may progress from residential to episomal, and, finally, to an integrated form. Residential infection typically occurs a minimum of 6 weeks from exposure, can persist without detection for decades, and can be low risk or high risk. In the episomal state, virally active HPV is located in the cell nucleus, separate from the human DNA. In the integrated form of infection, the HPV DNA circle has opened and joined the human DNA. Integrated HPV—always high risk—produces an abnormal Papanicolaou (Pap) test. If recognized on colposcopy, it must be treated to prevent progression to cervical cancer.
FIGURE
How HPV infection progresses to cervical cancer
Adapted with permission from Pinto and Crum 200023 and Schlecht et al 2001.24
TABLE
Factors that put women at risk for HPV infection
| Young age (peak age group: 20–24 years) |
| Lifetime number of sexual partners |
| First sexual intercourse at early age |
| Male partner sexual behavior |
| Smoking |
| Oral contraceptive use |
| Uncircumcised male partners |
| Sources: Winer et al 2003;8 Schiffman and castle 2003;14 Insinga et al 2003.15 |
Why screening alone isn’t enough
New technologies for Pap testing, HPV DNA testing, and revisions in the Bethesda system for reporting cervical cytology have led to better treatment recommendations for patients with abnormal cytology results.26 But despite these advances, cervical screening is underused or not used at all for many women at risk.
For example, some women with abnormal cervical cytology—especially those of lower socioeconomic status, who often are medically underserved or lack insurance—may not receive adequate follow-up care.27 Though widespread cervical screening in the future may significantly decrease morbidity and mortality associated with cervical cancer, HPV vaccination can also help achieve this goal.
The case for vaccination plus screening
It will likely take at least a decade to assess the impact of HPV vaccination on invasive cervical cancer, and perhaps 20 to 30 years to achieve the maximum benefit from such a program. A computer-based model of the natural history of HPV and cervical cancer developed by the Harvard School of Public Health considered different cancer prevention policies, including vaccination against HPV types 16 and 18 (initiated at the age of 12 years), cytologic screening (initiated at 18, 21, 25, 30, or 35 years,) and combined vaccination and screening strategies. The model showed the combination strategy to be most effective.28