Prandial insulin is as effective as carbohydrate counting
If a patient doesn’t reach his or her A1c targets despite appropriate titration of the basal insulin dose, injections of a rapid-acting insulin analog at mealtime may be necessary. You can add rapid-acting insulin to the regimen, starting with 1 injection at the largest meal of the day and then adding an injection at additional meals as needed.
- A typical starting dose of rapid-acting prandial insulin (insulin aspart [NovoLog], insulin glulisine [Apidra], or insulin lispro [Humalog]) would be 5 to 10 U per meal.29
- The range of daily dose, when used at 3 meals per day, would be 0.2 to 0.5 U/kg per day (ie, 0.1 to 0.15 U/kg per meal).29 For a patient who weighs 100 kg, that would mean 20 U (6–7 units before each meal) per day.
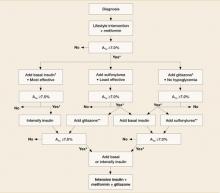
FIGURE
An evidence-based algorithm for achieving normal glycemic goals in patients with type 2 diabetes
Note: Reinforce lifestyle intervention at every visit.
*Check A1c every 3 months until <7% and then at least every 6 months.
†At A1C >9%.
‡At A1C ≤8%.
**Although 3 oral agents can be used, initiation and intensification of insulin therapy is preferred based on effectiveness and expense.
Reprinted with permission from the American Diabetes Association.13
Incretin mimetics
Incretins, such as glucagonlike peptide-1 (GLP-1), enhance glucose-dependent insulin secretion by the pancreatic beta cells and exhibit other antihyperglycemic actions after release into circulation by the gut.
Exenatide (Byetta) is the first agent in this class to be approved by the Food and Drug Administration for use in type 2 diabetes. Exenatide may be used in combination with oral therapy (a sulfonylurea and/or metformin) by patients who have not achieved adequate glycemic control on oral therapy alone. Exenatide mimics the antihyperglycemic actions of GLP-1 but maintains a prolonged duration of action compared with endogenous GLP-1.15 This agent effectively addresses postprandial hyperglycemia by restoring a rapid postprandial insulin response; consider it when reduction of postprandial glycemic excursions is required.
When injected twice daily (before morning and evening meals), exenatide reduces hyperglycemia and promotes satiety, which in turn reduces caloric intake and body weight. In a recent study, exenatide achieved reductions in A1c (1.11%) similar to those observed with insulin glargine when it was added to sulfonylurea/metformin combination therapy for patients with type 2 diabetes.16 Exenatide reduced fasting plasma glucose (FPG) to a greater degree, whereas insulin glargine had a greater effect on FPG. This suggests that a basal insulin may be more appropriate when FPG levels are elevated (ie, patients with A1c levels >8.0%) and exenatide may be more useful for patients with A1c levels <8.0%, when elevated PPG levels are predominant.17 However, some patients with A1c levels >8.0% also may benefit from such intervention.
Liraglutide, another incretin mimetic, is in development for the treatment of type 2 diabetes. Liraglutide is a long-acting GLP-1 analog in phase 3 development for once-daily treatment of type 2 diabetes. The mechanism of action is similar to that of exenatide, but with a longer duration of action. Liraglutide may be suitable for once-daily administration. Initial data indicate that liraglutide improved glycemic control while providing modest weight loss for patients with type 2 diabetes.18 (Available online at: www.novonordisk.com/science/pipeline/rd_pipeline.asp. Accessed August 2, 2007.)
DPP-IV inhibitors
A new class of oral antidiabetic agents, DPP-IV inhibitors slow the degradation of incretin hormones, allowing these hormones to stimulate insulin secretion and decrease glucagon levels in the circulation in a glucose-dependent manner.19,20
Sitagliptin (Januvia) has been approved for use as monotherapy or in combination with metformin, pioglitazone, or rosiglitazone for the treatment of type 2 diabetes. Once-daily sitagliptin improves glycemic control, reducing A1c from 0.4% to 1.1% and decreasing fasting plasma glucose from 12 to 17 mg/dL and 2-hour postprandial glucose from 49 to 62 mg/dL in clinical trials. It is well-tolerated,21,22 but its long-term efficacy and safety are unknown.
Vildagliptin (Galvus), another DPP-IV inhibitor has completed phase 3 testing and is pending FDA approval at this time. (Available online at: www.diabeteshealth.com/read/2007/03/16/5039.html. Accessed August 2, 2007.)
Recent data indicate that a simple treatment algorithm based on preprandial glucose patterns can be as effective as carbohydrate counting for the dose titration of prandial insulin.30 In this 24-week study, insulin glulisine was added to basal-prandial insulin therapy, with insulin glargine as the basal insulin component. Glulisine was adjusted to target using either a simple algorithm of adding 1, 2, or 3 U based on premeal glucose patterns or standard carbohydrate counting. The carbohydrate counting–based dose adjustment and the algorithm-based titration treatment arms achieved similar A1c reductions. However, patients using the simple algorithm experienced significantly less symptomatic hypoglycemia (P=.02).