Type 2 diabetes (T2D) is characterized by altered glucose homeostasis, including decreased insulin sensitivity of target tissues, a gradual decline in β-cell insulin production and secretion, and a progressive inability to suppress pancreatic α-cell glucagon secretion.1 In the past, goals for therapy have focused primarily on insulin secretion, sensitization, and replacement. However, newer T2D medications utilize the incretin gut hormone pathway, a focus of scientific and clinical research for decades.2 The so-called insulin effect, known today as the incretin effect3 —ie, greater insulin secretion in response to nutrient ingestion—was identified in 1964 when Elrick et al4 demonstrated that orally administered glucose produced a significant and sustained increase in plasma insulin, whereas intravenously administered glucose produced a smaller and transient insulin increase. This finding was paramount in bringing incretin-based therapies to clinical practice.
The 2 most well characterized incretin hormones are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) ( Figure 1 ). Currently, therapeutic agents, acting as either an incretin mimetic (via GLP-1 analogs) or to inhibit the breakdown of GLP-1 (via dipeptidyl peptidase-4 [DPP-4] inhibitors) are available for treatment.2,3 Various DPP-4 inhibitors are in development, and 2 are approved by the US Food and Drug Administration (FDA): sitagliptin and the recently approved DPP-4 inhibitor, saxagliptin, are indicated for use in a broad range of patients, including those who are drug naïve or who have inadequate glycemic control on another oral antidiabetic drug (OAD). Both agents are approved as monotherapy and as an add-on to current antihyperglycemic therapy (ie, metformin [MET], sulfonylurea [SU], thiazolidinedione [TZD]), and are also approved as initial combination therapy with MET.5-7 Another DPP-4 inhibitor, alogliptin, failed to gain approval from the FDA, which indicated the need for additional data (Takeda Pharmaceutical Company Limited) ( Table 1 ).
Figure 1
DPP-4 Inhibitors: Mechanism of Glucose Control28
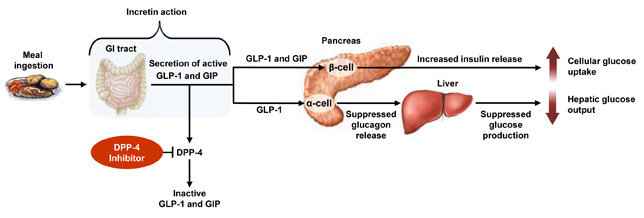
DPP-4, dipeptidyl peptidase-4; GI, gastrointestinal; GIP, glucose-dependent insulinotropic polypeptide; GLP-1, glucagon-like peptide-1.
Post–meal ingestion, GLP-1 and GIP are released from the small intestine and are rapidly degraded by the enzyme DPP-4. Inhibition of DPP-4 prevents the breakdown of GLP-1 and GIP and enhances glucose-stimulated insulin secretion (incretin action). GLP-1 and GIP act on the pancreatic β-cell to increase insulin release. GLP-1 also acts on the α-cell to suppress glucagon release and ultimately suppress hepatic glucose production. Together, the increased cellular glucose uptake and the decreased hepatic glucose output offer physiologic glucose control.
Table 1
DPP-4 Inhibitor Status and Availability
| Drug | Status | Trade name | Pharmaceutical Company |
|---|---|---|---|
| Alogliptin | Failed to gain approval | Not officially disclosed | Takeda Pharmaceutical Company Limited |
| Dutogliptin | Phase 3 | Not officially disclosed | Phenomix/Forest Laboratories, Inc. |
| Linagliptin | Phase 3 | Ondero® | Boehringer Ingelheim |
| Saxagliptin | Approved in the United States and Europe | Onglyza™ | Bristol-Myers Squibb/AstraZeneca |
| Sitagliptin | Approved in the United States and Europe | Januvia™, Janumet® | Merck & Co., Inc. |
| Vildagliptin | Approved in Europe | Galvus® | Novartis AG |
| DPP-4, dipeptidyl peptidase-4. | |||
The current therapeutic options for treating type 2 diabetes (T2D) include drug classes that lower blood glucose levels by different mechanisms of action ( Table ) through various target organs.1,2
Table
Drug classes that lower blood glucose levels
| Agent(s) | Mechanism of Action |
|---|---|
| • Insulin • Sulfonylureas • Glinides | Insulin replacement/secretion |
| • Thiazolidinediones | Insulin sensitization |
| • Biguanides | Decrease of hepatic glucose output |
| • α-Glucosidase inhibitors | Delay of intestinal carbohydrate absorption |
| • DPP-4 inhibitors • GLP-1 analogs | Incretin enhancement/replacement with subsequent effects on insulin and glucagon secretion |
| DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1. | |
Glucagon-like peptide-1 (GLP-1) is known to enhance insulin release from the pancreatic β-cells and inhibit glucagon release through the α-cells in a glucose-dependent manner.3 In the fasted state, circulating levels of GLP are low but rise within minutes of meal ingestion. GLP-1 is released from the L cells of the small intestine within minutes of food consumption; however, incretin hormones are rapidly degraded by the enzyme dipeptidyl peptidase-4 (DPP-4).3 By inhibiting DPP-4, the DPP-4 inhibitors enhance the half-life of GLP-1 and glucose-dependent insulinotropic polypeptide (GIP), thereby augmenting their levels.1,4 Because the release of GLP-1 is glucose dependent, augmentation of GLP-1 by DPP-4 inhibition minimizes the risk for hypoglycemia, which proves to be clinically important in managing T2D.
The majority of antidiabetic agents act primarily by lowering fasting plasma glucose (FPG) (eg, sulfonylureas), whereas others act primarily by lowering postprandial glucose (PPG).5 DPP-4 inhibitors primarily have a postprandial effect but also show statistically significant reductions in fasting glucose levels.6 FPG and PPG are the essential components of lowering glycosylated hemoglobin (HbA1c), and PPG has a greater effect on lowering HbA1c at values <8.5%.7
References
1. Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131-2157.
2. Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213-226.
3. Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3:153-165.
4. Stonehouse A, Okerson T, Kendall D, et al. Emerging incretin-based therapies for type 2 diabetes: incretin mimetics and DPP-4 inhibitors. Curr Diabetes Rev. 2008;4:101-109.
5. Leiter LA, Ceriello A, Davidson JA, et al. Postprandial glucose regulation: new data and new implications. Clin Ther. 2005;27(suppl B):S42-S56.
6. Rosenstock J, Aguilar-Salinas C, Klein E, et al. Effect of saxagliptin monotherapy in treatment-naive patients with type 2 diabetes. Curr Med Res Opin. 2009;25:2401-2411.
7. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881-885.

