TABLE 1
EXCLUSION CRITERIA
|
Study design
This randomized controlled parallel-group trial consisted of 3 four-week phases: a Baseline Feeding phase, a Medication Reduction phase, and a Maintenance phase. Eligible individuals were stratified by baseline systolic blood pressure (SBP) (< 140 mm Hg versus 140 mm Hg) and baseline soluble fiber intake (less than 3 grams/day versus 3 to 6 grams/day). At the start of the baseline phase, participants were randomized to either an oats cereal treatment group (n = 45) or a low-fiber cereal control group (n = 43).
The cereal treatments were isocaloric and administered during all 3 phases of the study. Individuals in the oats group received a daily serving of 60 grams (approximately three fourths cup) Quaker Oatmeal (5.61 grams total dietary fiber, 3.25 grams soluble fiber, and 2.83 grams -glucans) and 77 grams (approximately one and one third cups) Quaker Oat Squares (6.07 grams total dietary fiber, 2.98 grams soluble fiber, and 2.59 grams -glucans). Individuals in the control group consumed 65 grams (0.5 cup) Malt-O-Meal Hot Wheat Cereal (2.32 grams total dietary fiber, 0.6 grams soluble fiber) and 81 grams (2 cups) Kellogg’s Crispix (1.2 grams total dietary fiber, < 0.5 grams soluble fiber).
Cereals were dispensed in unlabeled bulk containers to facilitate physician blinding. Remaining cereal was returned and weighed at each of the weekly or biweekly visits at our research clinic. Additionally, participants kept a daily cereal calendar that was reviewed by members of our research staff and used to help determine cereal compliance.
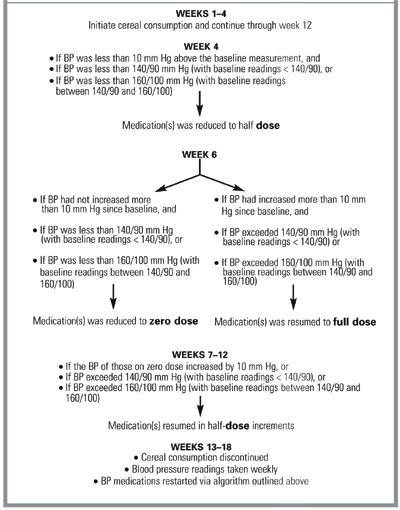
Changes in antihypertensive medication dose were implemented according to the protocol described in the Figure. Participants were asked to maintain their usual lifestyle, physical activity, dietary pattern, and body weight during the 12 weeks of the study. Individuals were invited to participate in a 6-week follow-up phase after the intervention was completed to monitor the residual BP effect after cereal consumption was discontinued.
FIGURE
MEDICATION REDUCTION PROTOCOL
Outcomes measured
The study physician responsible for BP measurement, blood draws, and general patient examinations (described below) was unaware of the cereal group assignment. BP was measured at the clinic twice a week during the first (baseline feeding) and last (maintenance) phases of the study and weekly during the second (medication reduction) phase. Participants reported at approximately the same time of the day for all appointments. BP readings were obtained 24 hours after the last medication dose or, if the patient was unmedicated, at the same time of day as previous study BP readings and after participants had rested quietly in the seated position for at least 5 minutes in an examination room.
The study physician took all readings on the right arm, using a mercury column sphygmomanometer (Korotkoff phase V for diastolic blood pressure [DBP]). Standard cuff size was used unless upper arm circumference exceeded 31 cm, in which case a large cuff with 15 x 35–cm bladders was chosen. Measurements were repeated 4 times in 2-minute intervals. The mean of the last 3 readings was calculated and used in subsequent analyses. Baseline and final study measurements used in the analyses and reported in this paper represent the averages of the first 2 and last 2 study visits.
Preintervention and postintervention blood samples were collected into standard 6-mL serum separator tubes. Samples were analyzed within 24 hours for general chemistry and plasma lipids (total cholesterol, low-density cholesterol [LDL-C], and high-density cholesterol [HDL-C] as well as triglyceride levels) by an accredited independent laboratory and according to standard chemical methods.16
A written 42-question side effect questionnaire was administered to participants at the beginning of the baseline phase and at the end of the intervention. Participants reported the frequency with which they experienced side effects associated with increased fiber intake (eg, loose stools, flatulence) and hypertension (eg, headaches, dizziness) using a 5-item scale ranging from “never” to “very frequently” (event occurring once or several times daily). Each item of the scale was assigned a value ranging from 1 to 5. Values were tallied across all 42 questions. A final score was assigned to each participant for both time points. Mean scores by group were used in the analyses.

