WASHINGTON – Endoscopically placed devices that include intragastric balloons and gastrointestinal liners are being evaluated for the treatment of obesity and could eventually fill some of the current void in treatment options that fall between lifestyle changes and surgical treatments.
These types of devices are the new paradigm for obesity treatments, and many are already available in Europe and elsewhere, Dr. Richard Rothstein said at a public workshop cosponsored by the Food and Drug Administration and the American Gastroenterological Association. Some have been evaluated in studies that use diabetes control measures as the main outcome endpoint, rather than weight loss, he noted.
Dr. Rothstein, the Joseph M. Huber Professor of Medicine and chair of the department of medicine, Geisel School of Medicine at Dartmouth, Hanover, N.H., provided an overview of some of the devices, which include sleeves, techniques, and devices used to reduce gastric volume such as balloons, and surgical tools used to sew or staple the stomach together.
Endoscopic treatments for obesity are less invasive, less expensive, reversible, and are performed as outpatient procedures, he said, noting that patients may be willing to pay for these procedures out of pocket.
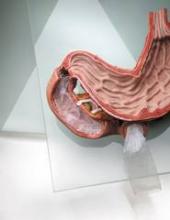
In early studies, the endoscopically delivered EndoBarrier resulted in weight loss and was well tolerated, said Dr. Rothstein, who is an adviser to the manufacturer, GI Dynamics. This is a flexible, tube-shaped liner that forms a physical barrier between food and the intestinal wall, according to the manufacturer. The device, which mimics malabsorptive surgical procedures, has been available for 3 years in Europe, Australia, and South America and is being evaluated in a pivotal U.S. study, the ENDO trial, for treating patients who are obese and have uncontrolled type 2 diabetes.
Andy Levine, who was chief technology officer at GI Dynamics and is now an adviser to the company, said that to date, the EndoBarrier, which completely covers the duodenum and the proximal jejunum and is anchored in the duodenal bulb distal to the pylorus, has been placed in more than 1,300 people worldwide. The device creates a "a complete bypass," so the food from the stomach goes through the flexible soft liner, driven by peristalsis, and the bile and pancreatic enzymes pass on the outside of the liner, with all mixing in the bowel, said Mr. Levine, who is now the leader of business development for medical devices and robotics at the Wyss Institute for Biologically Inspired Engineering at Harvard University, Boston.
In studies, improvements in type 2 diabetes measures are being used as endpoints, since showing improvement in obesity comorbidities was determined to be important for reimbursement, "and the technology had a unique impact on diabetes."
Based on experience with the device to date, Mr. Levine said that for the first 9-10 months, patients lose about 10%-20% of their body weight and many have been able to discontinue insulin and stay on oral therapy. Following removal of the device at 1 year, there is a slow rebound in weight and hemoglobin A1c values. While this might appear to be a temporary solution for a chronic problem, "we’re certainly treating it, and in some ways may be reversing the disease at least for awhile," he said, pointing out that the device addresses the need to break the cycle of continuing weight gain and helps patients discontinue diabetes medications, especially insulin.
A variety of newer, improved versions of endoscopically placed balloons are also being studied, including the ReShape, a double balloon device, Dr. Rothstein said.
During the same session, Richard Thompson, president and CEO of ReShape Medical, a small venture capital–funded company, said that the ReShape Duo intragastric device is made up of two balloons that contain a total of about 900 mL of fluid, which occupy space in the stomach without the discomfort and distension that can occur with one large balloon. This device is designed to reduce the risk of migration, which can occurs when a balloon deflates and migrates to the intestine where it can cause a blockage. If one balloon deflates the other remains inflated, and because there is blue dye in the balloons, when one deflates, the dye is visible in urine to alert the patient, he noted. ReShape Duo has been available in Europe for more than 2 years.