COEUR D’ALENE, IDAHO – Daily use of an over-the-counter 2-minute body wash by children and adolescents with moderate to severe atopic dermatitis resulted in marked improvement in symptoms and quality of life measures, as well as impressive changes in the skin microbiome in an open-label study.
"CLn Body Wash represents a simple and effective alternative to bleach bath use for patients who have colonization by Staphylococcus aureus and who prefer showers over baths," Dr. Adelaide A. Hebert said in presenting the study results at the annual meeting of the Society for Pediatric Dermatology.
The body wash, marketed by TopMD Skin Care, is a skin-friendly gel cleanser containing sodium hypochlorite at a concentration of 0.006%.
S. aureus colonization is common on both lesional and nonlesional skin of atopic dermatitis patients. It triggers inflammation and increases severity of the skin disease.
"I view colonization by Staph as the bully of atopic dermatitis. It really pushes and annoys the disease, and that’s the way I explain it to parents," said Dr. Hebert, professor and director of pediatric dermatology at the University of Texas, Houston.
Dilute bleach baths have been shown to reduce S. aureus colonization and diminish atopic dermatitis disease severity. The typical formula is one-quarter cup of household bleach added to half a bathtub of warm water, yielding a 0.005% sodium hypochlorite solution. But many people don’t like taking baths. They prefer showers. In addition, bathtubs are uncommon in many areas outside of the United States, Dr. Hebert noted.
Ease and convenience was the impetus for the development of CLn Body Wash. The product is packaged in a 2.5-oz bottle. Patients lather a few squirts of the wash onto the skin, then rinse it off after 1-2 minutes.
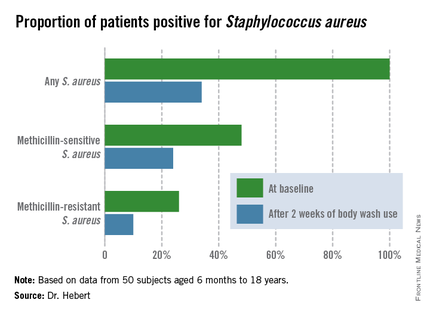
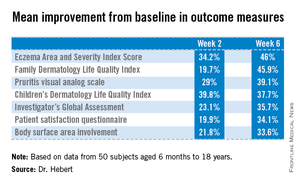
To evaluate the over-the-counter product’s effectiveness, Dr. Hebert and her coinvestigators conducted a 6-week, open-label, two-center study involving 50 subjects aged 6 months to 18 years, all with moderate to severe atopic dermatitis. Their mean age was 8 years. All participants were colonized by S. aureus, but none had an active infection. They were assessed at baseline, and again after 2 weeks and 6 weeks of once-daily use of the body wash.
Improvement was marked and consistent across numerous measures of disease severity and quality of life. In addition, there was a sharp reduction after 2 weeks of use in the proportion of patients who were skin-positive for S. aureus by PCR and a comparable decrease in those who were culture-positive. The prevalence of Staphylococcus aureus colonization was cut by two-thirds.
"All along the way we were able to evoke all the parameters of improvement simply by changing the colonization status of these patients. And that was very exciting," Dr. Hebert said.
But audience member Dr. Alfred T. Lane, recipient of the annual Alvin Jacobs Award at this year’s SPD meeting, noted that he found the study less than convincing.
"I’ve seen too many open-label studies that don’t work out. I really think that to have success, you want to have a placebo group. These data look fantastic. It looks extremely exciting, but without a placebo control, I’m a little bit skeptical, to be honest," declared Dr. Lane, emeritus professor of dermatology and of pediatrics at Stanford (Calif.) University.
The study was funded by TopMD Skin Care. Dr. Hebert reported having no relevant financial conflicts.