VIENNA – Patients with type 2 diabetes treated with the oral hypoglycemic saxagliptin (Onglyza) benefited from stabilization and, in some cases, reversal of urinary protein levels in a prespecified, secondary analysis of data collected from more than 16,000 patients in a randomized, international trial.
Progression of patients with diabetes from normoalbuminuria to microalbuminuria and then proteinuria tracks along with their deteriorating renal function. Until now, no agent has been identified that could stop or reverse this process, although a similar effect had been documented in results from a few prior, much smaller studies using other drugs from the class of dipeptidyl peptidase-4 (DPP-4) inhibitors, which includes saxagliptin.
“It’s a very important finding that needs further investigation in longer-term studies so we can see whether the improvement in the [albumin to creatinine ratio (ACR)] will also mean less deterioration of the eGFR [estimated glomerular filtration rate] and thereby prevent end-stage renal disease,” Dr. Ofri Mosenzon said following her report at the annual meeting of the European Association for the Study of Diabetes.
The new analysis also showed that saxagliptin did this safely, without causing any renal damage, and that the effect on microalbuminuria and proteinuria was completely independent of the drug’s glycemic control effect measured by hemoglobin A1c levels. Dr. Mosenzon had no simple explanation for the mechanism by which saxagliptin and other DPP-4 inhibitors might exert this effect, though she suggested that an anti-inflammatory pleiotropic effect of the drug class might be involved.
“We have this clinical result. Now we need to look for an explanation,” said Dr. Mosenzon, a diabetologist at Hadassah Hospital in Jerusalem.
Her analysis used data collected in the the Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus (SAVOR) – Thrombolysis in Myocardial Infarction (TIMI) 53 trial, which had the primary purpose of assessing the cardiovascular safety of saxagliptin in 16,492 patients with type 2 diabetes and a high risk for cardiovascular events (N. Engl. J. Med. 2013;369:1317-26). The study randomized patients to treatment with either 5 mg saxagliptin daily (2.5 mg daily in patients with impaired renal function at entry) or placebo in addition to whatever other standard medications their physicians prescribed. The secondary analysis of the impact of saxagliptin on proteinuria and renal function used prespecified definitions for evaluating the renal safety and renal-protective efficacy of treatment.
On the safety side, treatment with saxagliptin, compared with placebo linked with no statistically significant differences in the rate or magnitude of serum creatinine increases or in the rates of progression to dialysis or renal transplant.
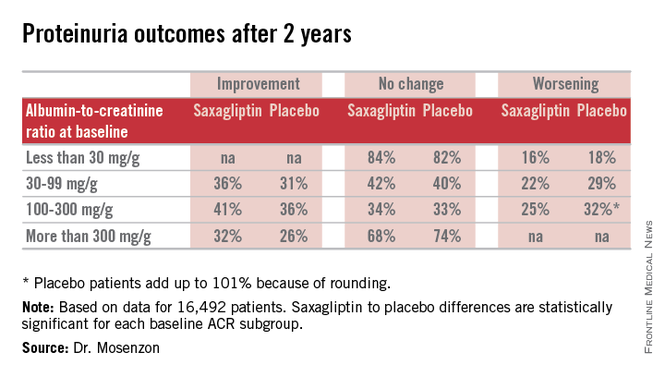
For efficacy, treatment with saxagliptin, compared with placebo led to a consistent pattern of stabilization and reversal of the severity of microalbuminuria and proteinuria that cut across patients at all baseline levels of urinary protein (see table). For example, among 1,638 categorized as having proteinuria at entry into the study, based on an ACR of more than 300 mg/g, 32% of the patients treated with saxagliptin had improved to daily urinary protein excretions of less than 300 mg/g after an average 2.1 years on treatment, compared with a 26% rate in the placebo-treated control patients, a statistically significant difference. The extent of reversals and progressions prevented were also statistically significant among the 9,696 patients who entered the study with normoalbuminuria and urinary protein levels of less than 30 mg/g and those who entered with microalbuminuria and daily urinary protein levels of 30-300 mg/g.
Expressed another way, when compared with placebo-treated patients those patients treated with saxagliptin for 2 years who entered the study with an eGFR of more than 50 mL/min/1.73 m2 had on average 19 mg less daily protein in their urine, those who entered with an eGFR of 30-50 mL/min/1.73 m2 averaged 105 mg less daily urine protein, and those who entered with an eGFR of less than 30 mL/min/1.73 m2 averaged 245 mg less daily urine protein.
SAVOR-TIMI 53 was sponsored by AstraZeneca and Bristol-Myers Squibb, the companies that market saxagliptin (Onglyza). Dr. Mosenzon has been a speaker for and advisor to those and to several other drug companies.