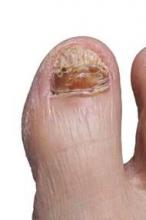
LAS VEGAS – The latest topical therapies for onychomycosis are strong enough to penetrate nail polish, but patients report few side effects.
“New topical antifungals have improved the challenge of nail penetration and enhanced the efficacy of topical therapy,” Dr. David Pariser said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar.
Two drugs, efinaconazole and tavaborole, have shown effectiveness against onychomycosis in clinical trials since 2013, and both have been approved by the Food and Drug Administration, said Dr. Pariser, a dermatologist in private practice in Norfolk, Va., and a professor at Eastern Virginia Medical School, Norfolk.
Significantly more patients in studies of both medications showed improvement after 52 weeks of treatment, compared with control patients, but the clinical trials of the two drugs aren’t directly comparable for several reasons, Dr. Pariser noted. The studies differed in their entry criteria, including patient age and nail involvement, and, in some cases, nails were clipped prior to clinical evaluation. In addition, the investigators in the studies used different laboratories, and the definitions of treatment success and investigators’ interpretations of clinical cure may vary, he noted.
Reports of adverse events related to the drugs were low for both. Although 65% of 801 patients in a pooled analysis of efinaconazole data reported at least one adverse event, only 8% of these events were deemed related to the study drug. No serious drug-related adverse events were reported in an analysis of 1,186 patients treated with tavaborole who reported at least one treatment-related adverse event.
The most common adverse events in efinaconazole patients included ingrown toenails, application-site dermatitis, and application-site vesicles, reported in approximately 2% of patients, and application-site pain reported by 1%. Similarly, the most common adverse events in tavaborole patients included application site exfoliation and ingrown toenail in approximately 3% of patients, application site erythema in 2%, and application site dermatitis in 1%.
In addition, early findings from separate studies of both medications suggest that their penetration ability is not affected by nail polish, although more research is needed, Dr. Pariser said.
Dr. Pariser has served as an investigator and consultant for Valeant and an investigator for Anacor. SDEF and this news organization are owned by Frontline Medical Communications.