Malignant mesothelioma poses a significant challenge for clinicians because of its ability to resist chemotherapy, but the use of three-dimensional tumor spheroid models has shown that local administration of paclitaxel in a nanoparticle platform achieved better tumor penetration than conventional paclitaxel therapy, investigators reported. The study is in the May issue of the Journal of Thoracic and Cardiovascular Surgery.
Dr. Hongyi Lei of Brigham and Women’s Hospital, Boston, and his colleagues used the in vitro mesothelioma spheroid model because two-dimensional in vitro monolayer cell culture experiments do not replicate the superior efficacy of paclitaxel-loaded expansile nanoparticles (Pax-eNPs), suggesting that Pax-eNPs utilize a unique drug delivery mechanism.
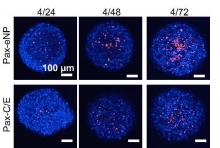
The study observed that spheroids treated with Pax-eNP showed increased drug penetration and a 38-fold higher intraspheroidal drug concentration at 24 hours than that of paclitaxel dissolved in Cremophor EL/ethanol (J. Thorac. Cardiovasc. Surg. 2014 [doi:10.1016/j.jtcvs.2015.02.020]).
The researchers said their findings showed that three-dimensional spheroid models “are valuable tools for investigating cytotoxic mechanisms and nanoparticle-tumor interactions, particularly given the costs and limitations of in vivo animal studies.” Their findings were first presented at the 94th Annual Meeting of the American Association of Thoracic Surgery last year in Toronto.
Despite advances of nanoparticle-based drug delivery systems, difficulties in evaluating the effectiveness of these drugs in local chemotherapy have hindered their adoption in the clinic. Studies of the same agent utilizing in vitro vs. in vivo methods have shown conflicting results.
The observation that Pax-eNP treatment of intraperitoneal mesothelioma significantly improved survival in lab animals in vivo compared to conventional paclitaxel led to the use of the three-dimensional spheroid model. Dr. Lei and colleagues called this revelation “striking” because Pax-eNP exposure of the identical mesothelioma tumor cells plated as a two-dimensional monolayer in vitro demonstrated equal or worse results. “This suggested that eNP may be more effective at penetration and/or persistence within multicellular tumors and led to the use of a 3-D tumor spheroid mode,” they said.
“Given the high cost and limitations of in vivo animal studies, spheroid models may present a clinically relevant platform for screening novel pharmaceuticals and unique drug-delivery systems during the preclinical phase,” the researchers indicated.
They also investigated spheroid cytotoxicity in a clinic-like setting following a 4-hour, high-dose (1,000 ng/mL) paclitaxel exposure via conventional and eNP vehicles. They found that Pax-eNP exposure led to greater tumor cytotoxicity at 72 hours, and that cytotoxicity continued seven days later because Pax-eNPs rapidly enter the tumor spheroid and remain intracellular, slowly releasing the drug.
“The prolonged drug release mechanism that pH-triggered Pax-eNP uses appears to be unique, leading to markedly higher intraspheroidal drug delivery, prolonged intratumoral drug release and superior antitumor efficacy,” the investigators concluded. The authors had no disclosures.