Medication-assisted therapies
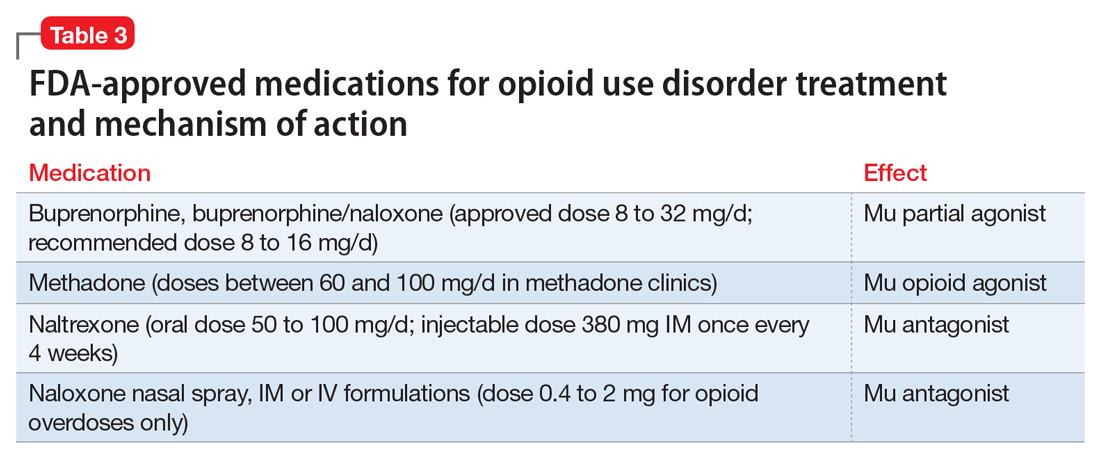
Detoxification alone often is not sufficient treatment. Medication-assisted therapy (MAT) is typically recommended by federal guidelines provided by the Substance Abuse and Mental Health Administration (SAMHSA) for patients with opioid use disorders.3 Patients can be directly transitioned from currently abused opioids to MAT on an outpatient basis. FDA-approved medications for MAT for opioid use disorder include buprenorphine, naltrexone (oral and long-acting injectable), and methadone (Table 3). Choice of MAT depends on several factors, including cost, patient preference, and availability of methadone programs and buprenorphine providers.28
MAT should include psychosocial support29-33 and active monitoring with urine drug screens. Maintenance therapy with medications is usually long-term and has been shown to have better outcomes than detoxification alone or short-term treatment.34 Relapse during MAT should not be cause to discontinue treatment; instead, the patient should be referred to a higher level of care.
Some patients require individualized treatment approaches. For example, the SAMHSA has developed specific treatment improvement protocols to tailor treatments to address specific needs of adolescents.32 The American Academy of Pediatrics recommends MAT with buprenorphine in adolescents with opioid use disorder.33 Although methadone has been approved for pregnant, opioid-dependent patients, recent data indicate buprenorphine is as effective with lower intensity of neonatal abstinence syndrome.34
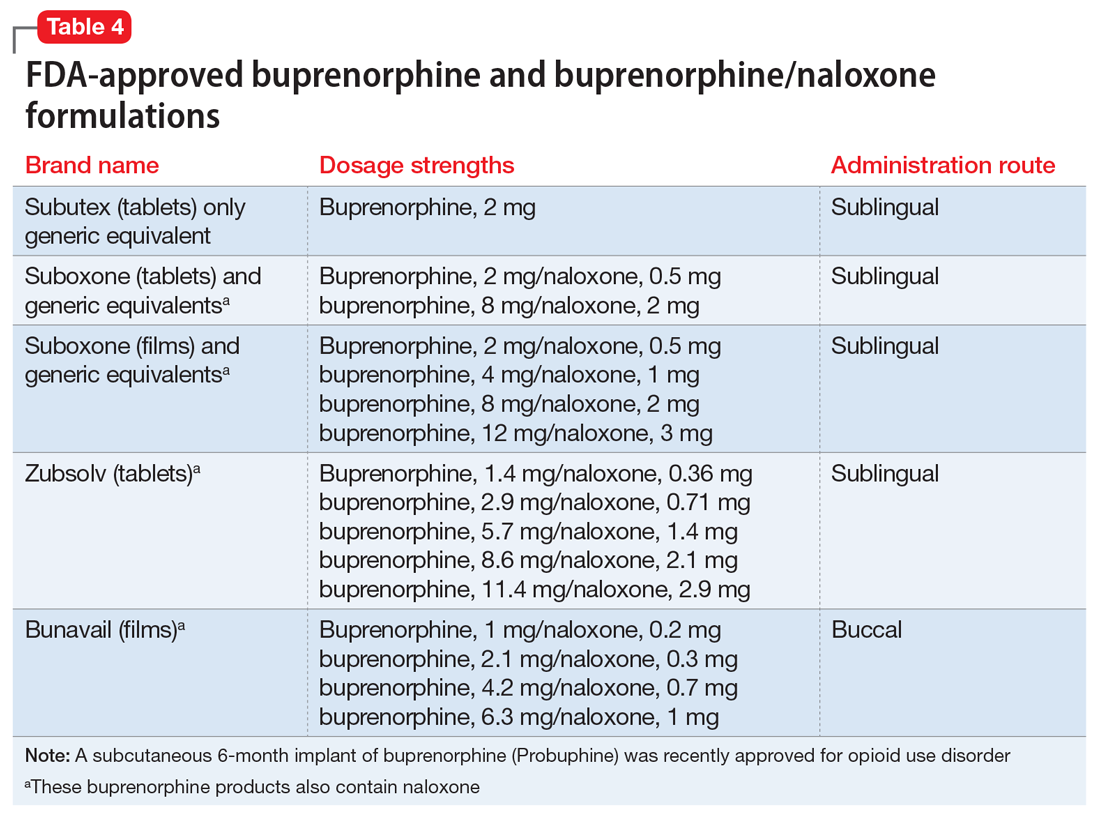
Buprenorphine. This long-acting (half-life of 24 to 42 hours) opioid partial agonist is approved for treating opioid use disorder in office-based settings according to the Drug Abuse Treatment Act of 2000. Buprenorphine is administered in doses of 8 to 16 mg/d in film or tablet form (sublingual or buccal) and is available in various formulations (Table 4). It is well tolerated; constipation and unpleasant taste are the most common adverse effects. Physicians are required to have a federal waiver to obtain the Drug Enforcement Administration license to prescribe buprenorphine for opioid use disorder in an office setting.
Buprenorphine reduces or eliminates cravings and withdrawal symptoms and helps improve outcomes of abstinence from opioids and retention in treatment.31 Formulations of naloxone combined with buprenorphine reduce the risk of abuse via injection.35 Buprenorphine is safe; however, overdoses can occur when it is combined with benzodiazepines and/or other opiates.
Methadone. This long-acting (half-life 8 to 59 hours), full opioid agonist is approved to treat opioid addiction in federal- and state-regulated opioid treatment programs, also known as methadone maintenance programs. These programs are highly structured and include intensive counseling, monitoring, and dispensing to reduce relapse. Methadone is administered orally either via powder, liquid concentrate, tablet, or solution of diskette. Typically, methadone is dispensed daily in doses of 60 to 100 mg, although higher doses are sometimes necessary. Patients who meet certain criteria for stability may be allowed to take home supplies of methadone.
Methadone has a “black-box” warning for overdose, QT prolongation, and risk for respiratory depression when used in combination with benzodiazepines. Because of its long and unpredictable half-life and tissue accumulation, methadone carries a high overdose risk, particularly with rapidly titrated doses during therapy initiation.35 However, most overdose deaths have occurred with methadone prescribed for pain management. When prescribed and monitored in an opioid treatment program, methadone has shown a high safety profile with respect to overdoses.36
Injectable and oral naltrexone. Used for prevention of relapse to opioid dependence, naltrexone is a pure opioid antagonist that is available as an oral or IM form. Naltrexone has high affinity for the opioid receptors and in therapeutic doses provides an effective blockade for heroin or opioids. Compliance with oral naltrexone has been poor, leading to development of an IM form of naltrexone that can be administered as a single 380-mg dose once every 4 weeks for 6 months or sometimes longer. Naltrexone is also approved for alcohol dependence.
To avoid precipitated withdrawal, patients should be detoxified from opioids for 7 to 10 days before they begin naltrexone, which has no potential for abuse. Common adverse effects include fatigue, nausea, headache, and, for the IM formulation, injection site reactions. There is a “black-box” warning for liver toxicity; therefore, baseline and periodic liver function tests are necessary.
A NIDA review reported poor compliance with oral naltrexone compared with methadone.35 However, naltrexone has been shown to be effective in highly motivated patients (eg, impaired physicians) and the criminal justice population and for preventing relapse following taper from buprenorphine or methadone.37,38
Treatment for opioid overdose
Naloxone is a highly effective treatment to reverse opioid overdose that is delivered via IM or IV injection or by nasal application. Naloxone has no abuse potential. In doses of 0.4 to 2 mg, naloxone reverses overdose within 2 minutes and is effective for 30 to 90 minutes.39 One should call 911 as soon as possible after naloxone is administered. In several states, naloxone is available without a prescription for patients and family members to combat opioid overdoses. The CDC recommends offering naloxone to patients who have risk factors for opioid overdose.40