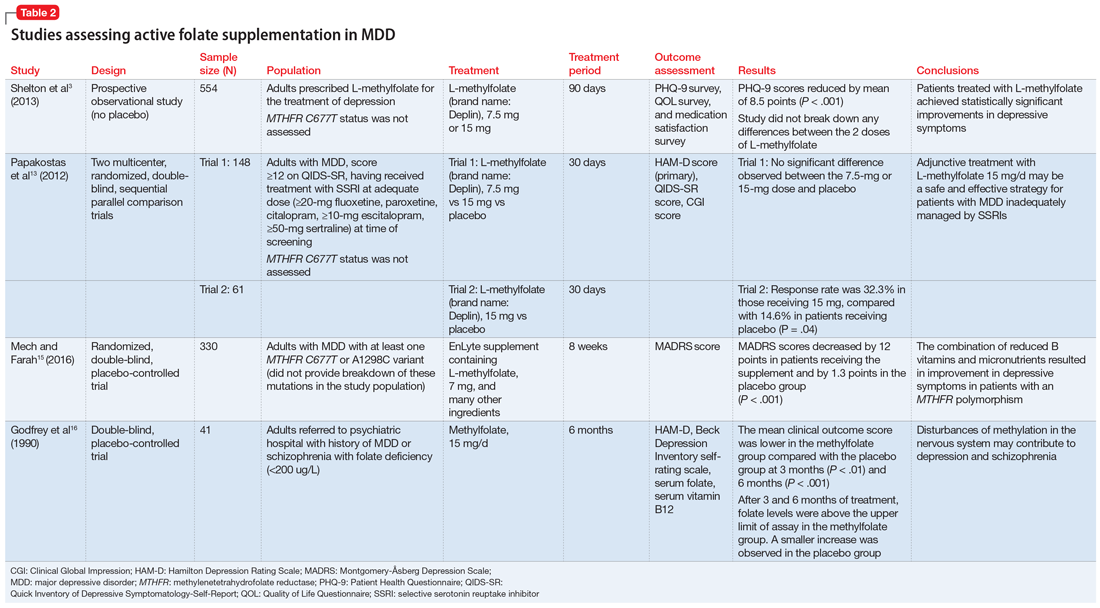
Mech and Farah15 performed a randomized, double-blind, placebo-controlled study of the use of EnLyte, a supplement containing 7-mg L-methylfolate, in patients with at least 1 variant of MTHFR (either C677T or A1298C) over an 8-week period. In addition to L-methylfolate, this supplement contains other active ingredients, including leucovorin (or folinic acid), magnesium ascorbate, and ferrous glycine cysteinate. Montgomery-Åsberg Depression Scale (MADRS) scores improved by 12 points in patients who received the supplement and by 1.3 points in patients who received placebo. However, because the supplement contained many ingredients, the response observed in this study cannot be attributed to L-methylfolate alone.15
Table 23,13,15,16 contains summaries of these and other selected studies assessing active folate supplementation in MDD.
CASE CONTINUED
Over the next several weeks, Ms. T experiences some modest improvement in mood while taking L-methylfolate and her antidepressant regimen, and she experiences no notable adverse effects. Unfortunately, after 3 months, Ms. T discontinues the supplement due to the cost.
The value of MTHFR testing
Ms. T’s case is an example of how clinicians may respond to MTHFR pharmacogenetic testing. Although L-methylfolate has shown some benefit in several randomized clinical trials, available data do not confirm the relevance of MTHFR functional status to symptom response. Additionally, there is likely interplay among multiple factors affecting patients’ response to L-methylfolate. Larger randomized trials prospectively assessing other pharmacogenetic and lifestyle factors may shed more light on which patients would benefit.
Based on available data, the decision to prescribe L-methylfolate should not necessarily hinge on MTHFR genetics alone. Both patients and clinicians must be aware of the potentially prohibitive cost if L-methylfolate is recommended, as prescription insurance may not provide coverage (eg, a recent search on GoodRx.com showed that generic L-methylfolate was approximately $40 for 30 tablets; prices may vary). Additionally, clinicians should be aware that L-methylfolate is regulated as a medical food product and is not subject to strict quality standards required for prescription medications. Future prospective studies assessing the use of L-methylfolate specifically in patients with a MTHFR variants while investigating other relevant covariates may help identify which specific patient populations would benefit from supplementation.
Continue to: Related Resources