The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
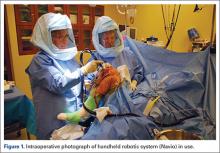
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40