PATIENT-SPECIFIC GLENOID COMPONENT
The Vault Reconstruction System ([VRS], Zimmer Biomet) is a patient-specific glenoid vault reconstruction system developed with the use of CAD/CAM to address severe glenoid bone loss encountered during shoulder arthroplasty. For several years, the VRS was available only as a custom implant according to the US Food and Drug Administration rules, and therefore its use was limited to a few cases per year. Recently, a 510(k) envelope clearance was granted to use the VRS in reverse TSA to address significant glenoid bone defects.
The VRS is made of porous plasma spray titanium to provide high strength and flexibility, and allows for biologic fixation. This system can accommodate a restricted bone loss envelope of about 50 mm × 50 mm × 35 mm according to the previous experience of the manufacturer in the custom scenario, covering 96% of defects previously addressed. One 6.5-mm nonlocking central screw and a minimum of four 4.75-mm nonlocking or locking peripheral screws are required for optimal fixation of the implant in the native scapula. A custom boss can be added in to enhance fixation in the native scapula when the bone is sufficient. To facilitate the surgical procedure, a trial implant, a bone model of the scapula, and a custom boss reaming guide are 3-dimensional (3-D) and printed in sterilizable material. These are all provided as single-use disposable instruments and can be available for surgeons during both the initial plan review and surgery.
PREOPERATIVE PLANNING
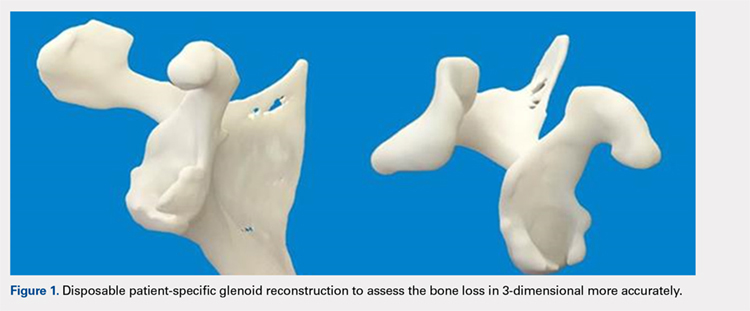
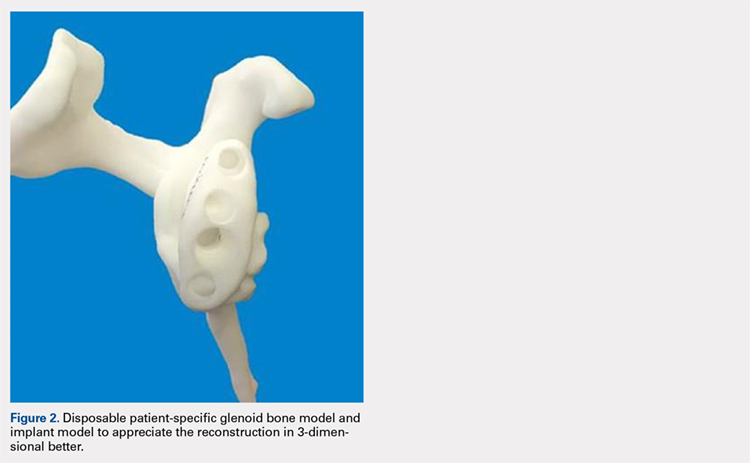
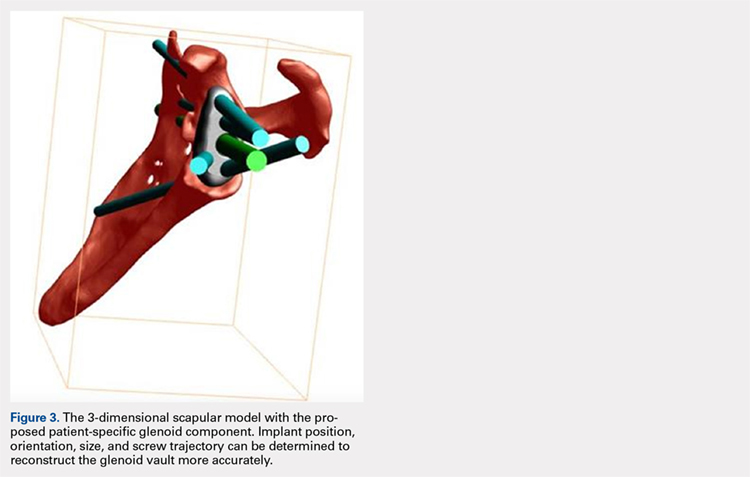
Patients undergo a preoperative fine-cut 2-dimensional computed tomography scan of the scapula and adjacent humerus following a predefined protocol with a slice thickness of 2 mm to 3 mm. An accurate 3-D bone model of the scapula is obtained using a 3-D image processing software system (Figure 1). The 3-D scapular model is used to create a patient-specific glenoid implant proposal that is approved by the surgeon (Figure 2). Implant position, orientation, size, screw trajectory, and recommended bone removal, if necessary, are determined to create a more normal glenohumeral center of rotation and to secure a glenoid implant in severely deficient glenoid bone (Figure 3). Once the implant design is approved by the surgeon, the final patient-specific implant is manufactured.
SURGICAL TECHNIQUE
The exposure of the glenoid is a critical step for the successful implantation of the patient-specific glenoid implant. Soft tissue and scar tissue around the glenoid must be removed to allow for optimal fit of the custom-made reaming guide. Also, removal of the entire capsulolabral complex on the anteroinferior rim of the glenoid is essential to both enhance glenoid exposure and to allow a perfect fit of the guide to the pathologic bone stock. Attention should be paid during débridement and/or implant removal in case of revision, to make sure that no excessive bone is removed because the patient-specific guide is referenced to this anatomy. Excessive bone removal can change the orientation of the patient-specific guide and ultimately the fixation of the implant. Once the custom-made patient-specific guide is positioned, a 3.2-mm Steinmann pin is placed through the inserter for temporary fixation. The pin should engage or perforate the medial cortical wall to ensure that the subsequent reamer has a stable cannula over which to ream. After the glenoid is reamed, the final implant can be placed in the ideal position according to the preoperative planning. A central 6.5-mm nonlocking central screw and 4.75-mm nonlocking or locking peripheral screws are required to complete the fixation of the implant in the native scapula. Once the patient-specific glenoid component is positioned and strongly fixed to the bone, the glenosphere can be positioned according to the preoperative planning, and the reverse shoulder arthroplasty can be completed in the usual fashion.
CASE EXAMPLES
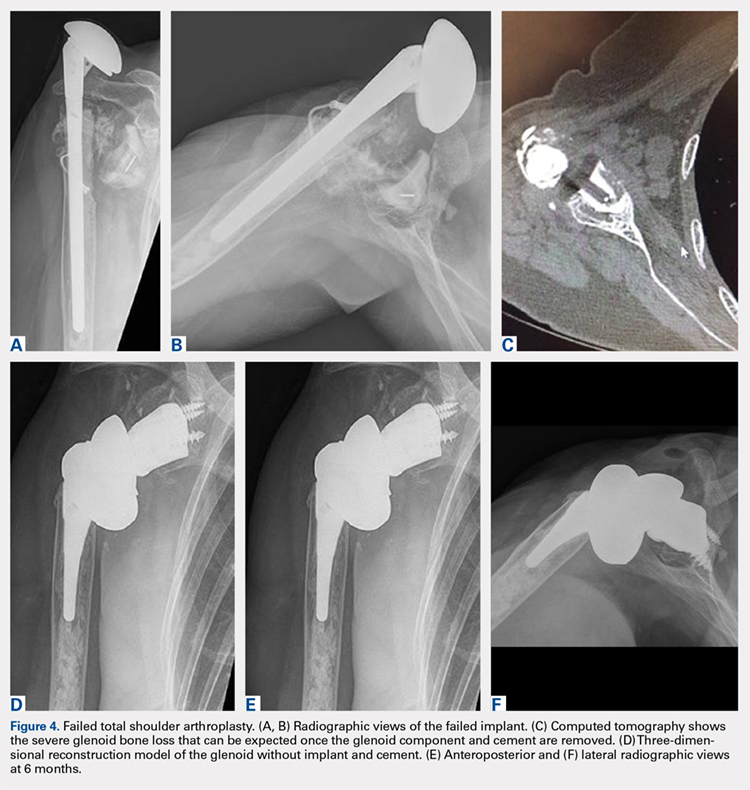
A 68-year-old woman underwent a TSA for end-stage osteoarthritis in 2000. The implant failed due to a cuff failure. The patient underwent several surgeries, including an open cuff repair, with no success. She had no active elevation preoperatively. Because of the significant glenoid bone loss, a patient-specific glenoid reconstruction was planned. Within 24 months after this surgery, the patient was able to get her hand to her head and elevate to 90º (Figures 4A-4F).
Continue to: In October 2013...