Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
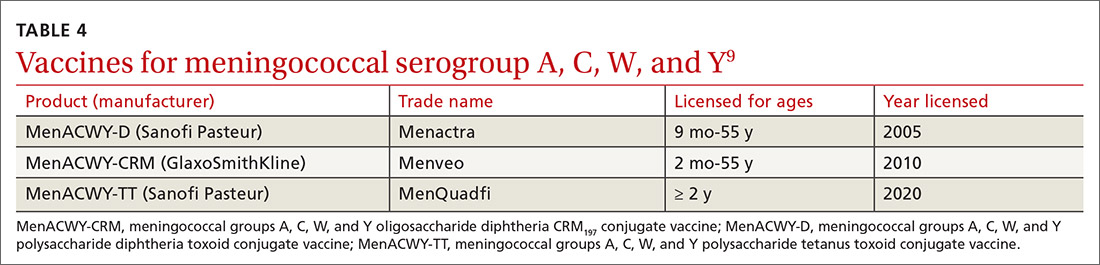
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
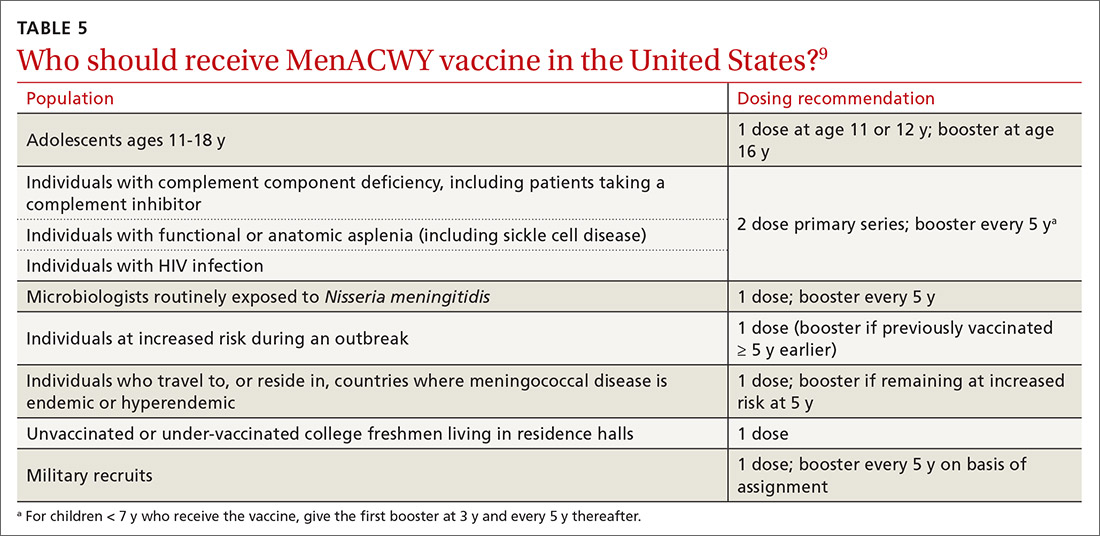
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
Continue to: Ebola virus (EBOV) vaccine