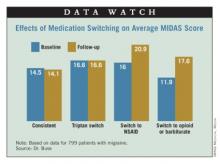
Results showed that headache-related disability, assessed from Migraine Disability Assessment (MIDAS) scores, was essentially unchanged from one year to the next in patients who stayed on the same triptan (14.5 vs. 14.1) or who switched from one triptan to another (16.6 vs. 16.6). In contrast, scores increased (worsened) in patients who switched to NSAIDs (16.0 vs. 20.9) and who switched to combination therapy containing opioids or barbiturates (11.9 vs. 17.6).
Additional analyses showed that the impact of switching to NSAIDs varied according to migraine frequency. Specifically, patients with high-frequency episodic migraines (10-14 headache-days monthly) or chronic migraines (15 or more headache-days monthly) had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines (0-4 headache-days monthly) or moderate-frequency episodic migraines (5-9 headache-days monthly).
"Not only did the treatment matter, but the average number of days of headache at baseline matters quite importantly here," Dr. Buse commented.
"In this observational study, switching triptan regimens or switching from a triptan to an alternative pharmacologic therapy for acute migraine does not appear to be associated with improvements in headache-related disability and, in some cases, is associated with increased headache-related disability over the course of 1 year to the second year," she concluded.
Adding medications
Dr. Lipton’s team studied the impact of medication additions from one year to the next in 960 patients with migraine taking triptans for acute treatment.
"Most people with migraine in the population use more than one acute treatment, and triptan users often use more than one triptan or use a triptan in combination with other medications, or even use combination products, such as Treximet [sumatriptan and naproxen], which contains a triptan and a nonsteroidal [anti-inflammatory drug]," he noted.
"By and large, we have not done much in terms of studying combination acute treatment in migraine, and certainly combining triptans has not been studied very often," he noted.
Most patients, 68%, did not change their acute treatment, while 13% added a combination analgesic containing opioids or barbiturates, 12% added another triptan, and 7% added an NSAID.
Results showed that among patients having low-frequency episodic migraines, adding another medication to their triptan did not significantly affect MIDAS score from one year to the next, regardless of the type of medication added.
Among patients with medium-frequency episodic migraines, adding an NSAID was associated with a significant decrease in MIDAS scores, whereas adding other medications did not affect scores.
But among patients with high-frequency episodic migraines or chronic migraines, MIDAS scores actually increased significantly with addition of an NSAID or another triptan. They were unaffected by addition of a combination analgesic containing opioids or barbiturates.
Further analyses confirmed that the impact of adding NSAIDs varied according to migraine frequency: Patients with high-frequency episodic migraines or chronic migraines had a significant increase in MIDAS scores when switching to NSAIDs relative to their counterparts with low-frequency episodic migraines.
"This is analogous to the finding in the Bigal paper [Headache 2008;48:1157-68] showing that NSAIDs are associated with an increased risk of transition to chronic migraine in people who have high-frequency episodic migraine," Dr. Lipton commented.
Dr. Lipton disclosed that he receives research grant support from the National Institutes of Health, the National Headache Foundation, and the Migraine Research Fund, and serves as a consultant or advisor to or has received honoraria from the American Headache Society and various pharmaceutical companies manufacturing drugs for migraine. Dr. Buse disclosed that she has received grant support and honoraria from Endo Pharmaceuticals and other pharmaceutical companies manufacturing drugs for migraine. The AMPP study is funded through a research grant to the National Headache Foundation from Ortho-McNeil Neurologics; additional analyses and manuscript preparation were supported through a grant from MAP Pharmaceuticals and Allergan to the National Headache Foundation.