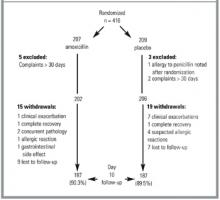
Complete or partial follow-up data were obtained for 374 patients (90%) after 10 days (mean 10.3 days, standard deviation 1.44): 334 patients completed the questionnaire, 348 returned the diary, and 338 underwent a physical examination. In 265 (71%) patients, data (questionnaire, diary, and physical examination) were complete; in 109 (29%), data at day 10 were partly missing. The two treatment groups were very similar in terms of sex, age, duration of preinclusion complaints, and frequency of various physical signs and symptoms (Table 1).*
TABLE 1
BASELINE CHARACTERISTICS
| General (placebo = 205, amoxicillin = 204) | Placebo | Amoxicillin |
|---|---|---|
| Mean age (SD) | 39 (15) | 37 (14) |
| Mean days of complaint before contact (SD | 7.2 (5.5) | 7.6 (5.4) |
| Women (%) | 54 | 55 |
| Mean Score on SNOT-20 (placebo = 196, amoxicillin = 192) | 40.8 (SD 15.9) | 38.4 (SD 16.1) |
| History (placebo = 196, amoxicillin = 192) | ||
| Generally ill to very ill (%) | 46 | 53 |
| Unilateral facial pain (%) | 56 | 53 |
| Pain on bending forward (%) | 70 | 66 |
| Pain in upper teeth or when chewing (%) | 44 | 41 |
| Examination (placebo = 209, amoxicillin = 207) | ||
| Sinus tenderness (%) | 61 | 67 |
| Pain on bending forward (%) | 60 | 60 |
| Postnasal discharge on throat inspection (%) | 55 | 50 |
| Purulent rhinorrhea on rhinoscopy (%) | 47 | 40 |
| Body temperature > 37°C (%) | 38 | 41 |
| SD denotes standard deviation; SNOT, Sino-Nasal Outcome Test. | ||
FIGURE 1
PATIENTS’ PROGRESS THROUGH THE TRIAL
Primary Outcomes
Of the 374 patients with follow-up data on day 10, 334 completed the symptom questionnaire twice. Treatment was successful—defined as a score of 0 (absent) or 1 (very mildly present) for all symptoms that had been included as “the most important item affecting my health”—in 35% of patients in the amoxicillin group (59/170) and 29% in the placebo group (47/164) (Table 2). Relative risk of success was 1.14 (95% CI, 0.92-1.42, P = .24): more patients were cured in the amoxicillin group, but this difference was not statistically significant.
In 82 (19.7%) of the 416 randomized patients (37 amoxicillin, 45 placebo), data on this main outcome are missing. In 40 of these 82 patients, follow-up data are available from the diary (n = 38) or physical examination (n = 2). According to these data, in 13/17 of the amoxicillin group and 11/23 of the placebo group the outcome was favorable: in the diary, the patient reports feeling “well” again at day 10 or sooner, or on physical examination, all signs of respiratory infection have cleared). Eight patients withdrew for clinical exacerbation and 2 patients after full recovery. Adding the 50 patients with a known course of illness to those in the treatment and result groups does not alter the overall result (RR 1.20, 95% CI, 0.98-1.47, P = .08). Furthermore, when considering the 24 nonexcluded patients (13 amoxicillin, 11 placebo) with total lack of follow-up in their allocated treatment group, first as treatment failures (RR 1.18, 95% CI, 0.97-1.44, P = .11) and then as successes (1.20, 95% CI, 0.99-1.46, P = .07), the result also remains the same. Regarding the success rate from the complete diary data (n = 348) and the results of physical examinations (n = 338) (Table 3), we find no significant difference between treatment groups.
Duration of purulent rhinorrhea was significantly shorter in the amoxicillin group than in the placebo group (75% of patients were free of purulent rhinorrhea after 9 days versus after 14 days in the placebo group, log rank P = .007). There is no difference between treatment groups in the duration of general illness or pain (Figure 2).
TABLE 2
MAIN OUTCOME: RATE OF TREATMENT SUCCESS AT 10-DAY FOLLOW-UP
| Outcome Measure | N* | Number with Successful Therapy (%) | Relative Risk of Success (95% CI) | p | |
|---|---|---|---|---|---|
| Amoxicillin | Placebo | ||||
| Survey† | 334 | 59/170 (35) | 47/164 (28) | 1.14 (0.92-1.42) | .24 |
| Diary ‡ | 348 | 92/174 (52) | 97/174 (55) | 0.94 (0.77-1.16) | .59 |
| Physical signs § | 338 | 97/170 (57) | 86/168 (51) | 1.13 (0.91-1.40) | .28 |
| All ║ | 384 | 73/189 (39) | 59/195 (30) | 1.2 (0.98-1.47) | .08 |
| Sensitivity analysis¶ | |||||
| Best case | 408 | 86/202 | 70/206 | 1.2 (0.99-1.46) | .07 |
| Worst case | 408 | 73/202 | 59/206 | 1.18 (0.97-1.44) | .11 |
| * Data on at least one of these outcome measures were obtained in 374 patients (90% of the total population). | |||||
| † All symptoms indicated by the patients at inclusion as “most important item affecting my health” score 0 (absent) or 1 (very mildly present) on day 10. | |||||
| ‡ Patient states in diary that he or she feels generally “well” again on day 10 or sooner. | |||||
| § All physical signs have disappeared at day 10 (pain on bending, sinus tenderness, postnasal drip, purulent rhinorrhea on rhinoscopy, elevated body temperature). | |||||
| ║Incorporating all available information from the questionnaire, diary, physical examination, and dropouts. | |||||
| Patients without data are considered, respectively, as treatment success (best case) or treatment failures (worst case). | |||||
TABLE 3
MEAN SYMPTOM CHANGE BETWEEN BASELINE AND 10-DAY FOLLOW-UP
| Mean Score Reduction | |||
|---|---|---|---|
| Symptom | Amoxicillin n = 170 | Placebo n = 164 | P * |
| Unilateral facial pain | 1 | 1.1 | .56 |
| Pain on bending forward | 1.21 | 1.32 | .55 |
| Pain in upper teeth or when chewing | 0.7 | 0.93 | .17 |
| Need to blow nose | 1.73 | 1.70 | .85 |
| Sneezing | 1.13 | 1.05 | .63 |
| Runny nose | 1.47 | 1.55 | .33 |
| Cough | 1.0 | 1.11 | .46 |
| Thick nasal discharge | 2.2 | 1.5 | < .0001 |
| Postnasal discharge | 1.29 | 1.09 | .26 |
| Ear fullness | 1.13 | 1.31 | .32 |
| Dizziness | 0.95 | 0.87 | .63 |
| Ear pain | 0.64 | 0.77 | .36 |
| Facial pain or pressure | 1.54 | 1.61 | .69 |
| Difficulty falling asleep | 1.14 | 1.26 | .54 |
| Wake up at night | 1.39 | 1.44 | .79 |
| Lack of a good night’s sleep | 1.24 | 1.44 | .28 |
| Wake up tired | 1.34 | 1.65 | .09 |
| Fatigue | 1.46 | 1.61 | .38 |
| Reduced productivity | 1.45 | 1.63 | .29 |
| Reduced concentration | 1.24 | 1.46 | .19 |
| Frustrated, restless, irritable | 0.87 | 1.41 | .91 |
| Sad | 0.38 | 0.52 | .18 |
| Embarrassed | 0.36 | 0.76 | .36 |
| * Student’s t test. | |||