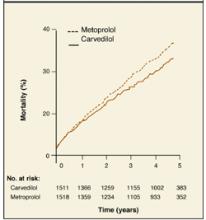
In the Carvedilol or Metoprolol European Trial (COMET),1 patients with heart failure were randomized to receive either carvedilol or metoprolol in addition to their current diuretic and angiotensin-converting enzyme inhibitor. A visual comparison of the survival curves shows a reduction in mortality in the carvedilol group compared with those in the metoprolol group (Figure 1).
Figure 1
Comparing survival curves
How to derive mortality reduction from survival curves
Different statistical methods are used to compare survival curves. Most commonly used is the hazard ratio, the increased speed with which one group is likely to experience an event at any given time in relation to another group. In the COMET trial, the hazard ratio for all-cause mortality was 0.83. This represents a 17% reduction in the risk of death with carvedilol compared with metoprolol.
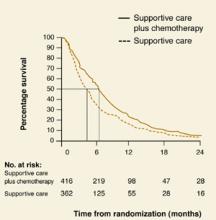
A rough estimate of the hazard ratio can be made by comparing median survival (the time point on each curve that corresponds to 50% survival) in both groups. In Figure 2, patients with non-small-cell lung cancer receiving supportive care (group A) had an approximate median survival of 4 months compared with 6 months in those who had also received chemotherapy (group B).
The hazard ratio is estimated by dividing the median survival time of group A by the median survival time of group B, or 4 months/6 months = 0.66. The reduction in risk of death for group B is therefore 37%. It is possible to estimate the hazard ratio this way only when the percent survival falls below 50% in each group.
Figure 2
Estimating hazard ratios
Correspondence
Mary K. Nordling, MD, Lawrence Family Practice Residency, 34 Haverhill Street, Lawrence, MA 01841. Email: mnordling@glfhc.org.