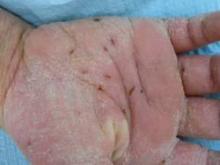
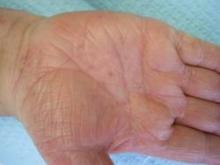
DENVER – A sodium hypochlorite wash significantly improved symptoms and quality of life for children who had atopic dermatitis with bacterial colonization, in a small study.
Staphylococcus aureus colonization occurs in the lesional and nonlesional skin of many atopic dermatitis patients, and bleach baths at a concentration of approximately 0.005% sodium hypochlorite are a common treatment, but not always an easy or convenient one, Dr. Benjamin R. Bohaty noted in a poster at the annual meeting of the American Academy of Dermatology.
In an open-label study, Dr. Bohaty of the University of Texas Health Science Center in Houston and his colleagues tested a gel cleanser containing a dilute concentration of 0.006% sodium hypochlorite wash that could be used in the bath or shower. The product is designed to be lathered onto the skin and rinsed off after 1-2 minutes. The study population included 40 children, average age 9 years, with a diagnosis of moderate to severe atopic dermatitis and S. aureus colonization. The children were instructed to use the wash once daily for 6 weeks, and they were assessed at three office visits during this period.
At 2 weeks and 6 weeks, the patients had improved an average of 34% and 44% from baseline, respectively, on the Eczema Area and Severity Index, and 23% and 34% from baseline on the Investigator’s Global Assessment scale score.
The average improvement in pruritus visual analog scale score was 31% at 2 weeks and 37% at 6 weeks.
Quality of life measures also improved during the study period. The average change in the Children’s Dermatology Life Quality Index was 41% at 2 weeks, dropping to 32% at 6 weeks, but both were significant improvements from baseline. The average improvement in the Family Dermatology Life Quality Index score was 13% at 2 weeks and 43% at 6 weeks, compared with baseline.
The significant decreases and lack of reported adverse events suggest that the wash is "a simple alternative to bleach baths," Dr. Bohaty reported.
The product is available in the United States under the label CLn Skin Care, a trademark of TopMD Skin Care, which funded the trial through research grants to the universities of the investigators.