Expert Commentary

Why FDA hearing on morcellation safety could drive innovation
Reaction from Cheryl Iglesia, MD, Advisory Panel Member, to FDA’s 2-Day Hearing
Q&A with Ray A. Wertheim, MD, and Harry Reich, MD
Dr. Wertheim is Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia.
Dr. Reich practices gynecologic surgery in Wilkes-Barre, Pennsylvania.
Dr. Wertheim and Dr. Reich report no financial relationships relevant to this article.

I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:

Reaction from Cheryl Iglesia, MD, Advisory Panel Member, to FDA’s 2-Day Hearing
Letters from readers, and a patient, on morcellation
The FDA’s recent safety communication on the risks of laparoscopic power morcellation prompts Brigham and Women’s and Massachusetts General...
James D. Kondrup, MD, demonstrates his surgical team's approach to morcellation, with use of a morcellation bag after laparoscopic supracervical...
Hospitals suspend use of power morcellators until further notice
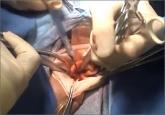
Ceana Nezhat, MD, and Erica Dun, MD, show how they perform enclosed vaginal morcellation
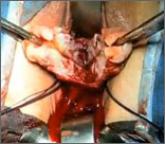
Dr. Shibley discusses a novel strategy he has developed to address the problem of tissue dispersion during open power morcellation
This technique is K. Anthony Shibley, MD's, short-term surgical solution to performing closed power morcellation. His patented pneumoperitoneum...
The most promising alternative to open power morcellation is morcellation in a bag, described here
