Practice Alert
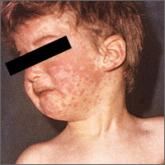
Measles: Why it’s still a threat
A recent outbreak in Minnesota underscores the need to maintain vigilance and adhere to best practices in immunization and containment of known...
Division of Infectious Diseases, Department of Pediatrics, University of Arkansas for Medical Sciences, Arkansas Children's Hospital, Little Rock
vvijayan@uams.edu
The author reported no potential conflict of interest relevant to this article.

Take into account routine childhood vaccinations, those that are mandatory for specific destinations, and these highly recommended immunizations.
› Recommend immunizations and safety precautions to international travelers based on their destinations, previous immunizations, health status and anticipated activities, and time available before departure. C
› Consider accelerating routine immunizations for children who may be traveling abroad. C
› Refer immunocompromised or pregnant patients to a travel medicine clinic for consultation before departure. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
International travel, whether for business, pleasure, child adoption, medical tourism, or adventure, continues to grow. In 2015, more than 70 million US citizens traveled internationally.1 Many individuals contact family physicians first about their plans for travel and questions about travel-related health advice. This article provides an overview of the vaccines recommended for travelers headed to international destinations. Because country-specific vaccination recommendations and requirements for entry and departure change over time, check the Centers for Disease Control and Prevention (CDC) Web site for up-to-date requirements and recommendations (www.cdc.gov/travel).
There is no single vaccination schedule that applies to all travelers. Each schedule should be individualized based on the traveler’s destination, risk assessment, previous immunizations, health status, and time available before departure.2,3 Pregnant or immunocompromised travelers should seek advice from an experienced travel medicine consultant on the immunization recommendations specifically meant for them.4,5
Travel vaccines (TABLE6) are generally categorized as routine, required, or recommended.
Pre-travel patient encounters are an opportunity to update routine vaccinations.7,8 Immunization against childhood diseases remains suboptimal in developing countries, where vaccine-preventable illnesses occur more frequently.9
Routine vaccines may be administered on an accelerated basis depending on geographic destination, seasonal disease variations, anticipated exposures, and known outbreaks at the time of travel.
MMR vaccine. Measles is still common in many parts of the world, and unvaccinated or incompletely vaccinated travelers are at risk of acquiring the disease and importing it to the United States (see “Measles: Why it’s still a threat,” 2017;66:446-449.) In 2015, a large, widespread measles outbreak occurred in the United States, linked to an amusement park in California, likely originating with an infected traveler who visited the park.10
Flu vaccine is recommended for all travelers ≥6 months of age, as flu season varies internationally.
All children older than 12 months should receive 2 doses of measles-mumps-rubella (MMR) vaccine separated by at least 28 days before departure (regardless of their destination). Infants between 6 and 11 months are at risk for high morbidity and may therefore receive a single dose of MMR earlier than the routinely recommended age of 12 to 15 months. Adolescents and adults without evidence of immunity against measles should get 2 doses of MMR separated by at least 28 days.11 Acceptable presumptive evidence of immunity against measles includes written documentation of adequate vaccination, laboratory evidence of immunity, laboratory confirmation of measles, or birth before 1957.
Varicella vaccine. Children, adolescents, and young adults who have received only one dose of varicella should get a second dose prior to departure. For children 7 to 12 years, the recommended minimum interval between doses is 3 months. For individuals 13 years or older, the minimum interval is 4 weeks.7,8
Influenza vaccine is routinely recommended for all travelers 6 months of age or older, as flu season varies geographically. Flu season in the Northern Hemisphere may begin as early as October and can extend until May. In the Southern Hemisphere, it may begin in April and last through September. Travelers should be vaccinated at least 2 weeks before travel in order to develop adequate immunity.12,13
Yellow fever (YF) is a mosquito-borne viral illness characterized by fever, chills, headache, myalgia, and vomiting. The disease can progress to coagulopathy, shock, and multisystem organ failure.14 YF vaccine is recommended for individuals 9 months or older who are traveling to or living in areas of South America or Africa where YF virus transmission is common (map: http://www.cdc.gov/yellowfever/maps/).
YF vaccine is a live-attenuated virus formulation and, therefore, should not be given to individuals with primary immunodeficiencies, transplant recipients or patients on immunosuppressive and immunomodulatory therapies, or patients with human immunodeficiency virus (HIV) whose CD4 count is below 200/mL. Other contraindications to YF vaccine are age younger than 6 months, allergy to a vaccine component, and thymic disorders. Serious adverse reactions to the vaccine are rare, but include 2 syndromes: YF-associated neurotropic disease and YF vaccine-associated viscerotropic disease.15
In many YF-endemic countries, vaccination is legally required for entry, and proof of vaccination must be documented on an International Certificate of Vaccination or Prophylaxis (ICVP). Additionally, some countries may require proof of vaccination before allowing travel through an endemic region, to prevent introduction of the disease elsewhere. Travelers with a specific contraindication to YF vaccine should obtain a waiver from a physician before traveling to a country requiring vaccination.16
The vaccination certificate is valid beginning 10 days after administration of YF vaccine. Immunity after a single dose is long lasting and may provide lifetime protection. Previously, re-vaccination was required every 10 years; however, in February 2015, ACIP approved a new recommendation stating a single dose of YF vaccine is adequate for most travelers.17
Many countries in which yellow fever is endemic don't allow entry without proof of vaccination on an International Certificate of Vaccination or Prophylaxis.
Although ACIP no longer recommends booster doses of YF vaccine for most travelers, clinicians and travelers should review the entry requirements for destination countries because changes to the International Health Regulations have not yet been fully implemented. Once this change is instituted, a completed ICVP will be valid for the lifetime of the vaccine.18,19 Country-specific requirements for YF can be found at http://www.cdc.gov/yellowfever/maps/. (Click on the link below the appropriate map.) In the United States, the YF vaccine is distributed only through approved vaccination centers. These designated clinics are listed in a registry on the CDC travel Web site at https://wwwnc.cdc.gov/travel/yellow-fever-vaccination-clinics/search.
Meningococcal disease. ACIP recommends routine vaccination against meningococcal disease for people 11 to 18 years of age and for individuals with persistent complement component deficiency, functional or anatomic asplenia, and HIV. Vaccination is recommended for travelers who visit or reside in areas where meningococcal disease is hyperendemic or epidemic, such as the meningitis belt of sub-Saharan Africa during the dry season of December to June (map: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/meningococcal-disease). Travelers to Saudi Arabia during the annual Hajj and Umrah pilgrimages are required to have a certificate of vaccination with quadrivalent (serogroups A, C, Y, W-135) meningococcal vaccine issued within 3 years (and not less than 10 days) before entry.
Several meningococcal vaccines are available in the United States. The quadrivalent vaccines are Menactra (MenACWY-D, Sanofi Pasteur) and Menveo (MenACWY-CRM, GSK). A bivalent (serogroups C and Y) conjugate vaccine MenHibrix (Hib-MenCY-TT, GSK) is also licensed for use in the United States, but infants traveling to areas with high endemic rates of meningococcal disease who received this vaccine are not protected against serogroups A and W and should receive quadrivalent meningococcal conjugate vaccine. Serogroup B vaccination is not routinely recommended for travelers. Approximately 7 to 10 days are required after vaccination for the development of protective antibody levels.7,8,20,21
Polio. Although polio has been nearly eradicated, as of the time this article was written, the disease has not been eliminated in Afghanistan, Guinea, Laos, Nigeria, or Pakistan. Other countries, such as Cameroon, Chad, and Ukraine remain vulnerable to international transmission.22 The CDC recommends that adults who are traveling to areas where wild polio virus (WPV) has circulated in the last 12 months and who are unvaccinated, incompletely vaccinated, or whose vaccination status is unknown should receive a series of 3 doses of IPV to prevent ongoing spread.23 Adults who completed the polio vaccine series as children and are traveling to areas where WPV has circulated in the last 12 months should receive a one-time booster dose of IPV.23
Infants and children in the United States should be vaccinated against polio as part of a routine age-appropriate series. If a child cannot complete the routine series before departure and is traveling to an area where WPV has circulated in the last 12 months, an accelerated schedule is recommended. Vaccination should be documented on the ICVP, as countries with active spread of poliovirus may require proof of polio vaccination upon exit. A list of the countries where the polio virus is currently circulating is available at http://polioeradication.org/polio-today/polio-now/wild-poliovirus-list/.
Both routine and accelerated vaccination schedules for children and adults are published annually by the CDC and are available at http://www.cdc.gov/vaccines/schedules/hcp/index.html.

A recent outbreak in Minnesota underscores the need to maintain vigilance and adhere to best practices in immunization and containment of known...
