Practice Alert
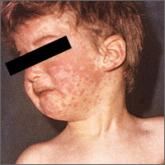
Measles: Why it’s still a threat
A recent outbreak in Minnesota underscores the need to maintain vigilance and adhere to best practices in immunization and containment of known...
Division of Infectious Diseases, Department of Pediatrics, University of Arkansas for Medical Sciences, Arkansas Children's Hospital, Little Rock
vvijayan@uams.edu
The author reported no potential conflict of interest relevant to this article.

Japanese encephalitis (JE) is endemic throughout most of Asia and parts of the Western Pacific region (map: http://www.cdc.gov/japaneseencephalitis/maps/). JE vaccine is recommended for travelers who plan to spend more than a month in endemic areas during the JE virus transmission season. (In temperate areas of Asia, JE virus transmission is seasonal and usually peaks in the summer and fall. In the subtropics and tropics, transmission can occur year-round, often with a peak during the rainy season.)
Adults who completed the polio vaccine series as children and are traveling where wild polio virus has circulated in the last 12 months should receive a one-time booster dose of IPV.
This recommendation includes recurrent travelers or expatriates who are likely to visit endemic rural or agricultural areas during a high-risk period of JE virus transmission. Risk is low for travelers who spend less than a month in endemic areas and for those who confine their travel to urban centers. Nevertheless, vaccination should be considered if travel is planned for outside an urban area and includes such activities as camping, hiking, trekking, biking, fishing, hunting, or farming. Inactivated Vero cell culture-derived vaccine (Ixiaro) is the only JE vaccine licensed and available in the United States. Ixiaro is given as a 2-dose series, with the doses spaced 28 days apart. The last dose should be given at least one week before travel.24
Typhoid fever. Vaccination against typhoid fever is recommended for travelers to highly endemic areas such as the Indian subcontinent, Africa, and Central and South America. Two typhoid vaccines are available: Vi capsular polysaccharide vaccine (ViCPS) administered intramuscularly (IM), and oral live attenuated vaccine (Ty21a). Ty21a is a live vaccine and should not be given to immunocompromised people or those taking antibiotics, as it may reduce immunogenicity. Ty21a must be kept refrigerated at 35.6° F to 46.4° F (2° C - 8° C) and administered with cool liquid no warmer than 98.6° F (37° C). Both vaccines are only 50% to 80% efficacious, making access to clean food and water essential.3,5,25
Hepatitis A vaccine should be given to all children older than one year traveling to areas where there is an intermediate or high risk of the disease. Children younger than one year who are traveling to high-risk areas can receive a single dose of immunoglobulin (IG) 0.02 mL/kg IM, which provides protection for up to 3 months. One 0.06 mL/kg-dose IM provides protection for 3 to 5 months.
If travel continues, children should receive a second dose after 5 months. IG does not interfere with the response to YF vaccine, but can interfere with the response to other live injected vaccines (such as MMR and varicella).26
Hepatitis B vaccination should be administered to all unvaccinated travelers who plan to visit an area with intermediate to high prevalence of chronic hepatitis B (HBV surface antigen prevalence ≥2%). Unvaccinated travelers who may engage in high-risk sexual activity or injection drug use should receive hepatitis B vaccine regardless of destination. Additionally, travelers who access medical care for injury or illness while abroad may also be at risk of acquiring hepatitis B via contaminated blood products or medical equipment.27
Serologic testing and booster vaccination are not recommended before travel for immunocompetent adults who have been previously vaccinated. The combined hepatitis A and B vaccine provides effective and convenient dual protection for travelers and can be administered with an accelerated 0-, 7-, and 21-day schedule for last-minute travelers.7,8
Rabies remains endemic in developing countries of Africa and Asia, where appropriate post-exposure prophylaxis is limited or non-existent.28 Consider pre-exposure rabies prophylaxis for traveling patients based on the availability of rabies vaccine and immunoglobulin in their destination area, planned duration of stay, and the likelihood of animal exposure (eg, veterinarians, animal handlers, cavers, missionaries). Advise travelers who decline vaccination to avoid or minimize animal contact during travel. In the event the traveler sustains an animal bite or scratch, immediate cleansing of the wound substantially reduces the risk of infection, especially when followed by timely administration of post-exposure prophylaxis.
In the event of an animal bite or scratch, immediate cleansing of the wound significantly reduces the risk of rabies infection.
Post-exposure prophylaxis for unvaccinated individuals consists of local infiltration of rabies immunoglobulin at the site of the bite and a series of 4 injections of rabies vaccine over 14 days, or 5 doses over one month for immunosuppressed patients. The first dose of the 4-dose course should be administered as soon as possible after exposure. Two vaccines are licensed for use in the United States: human diploid cell vaccine (HDCV, Imovax Rabies, Sanofi Pasteur) and purified chick embryo cell vaccine (PCECV, RabAvert, Novartis Vaccines and Diagnostics). The vaccine should never be administered in the gluteal area, as this may result in lower antibody titers.29
Additionally, promising new vaccines against malaria and dengue fever are under clinical development and may be available in the near future.
CORRESPONDENCE
Vini Vijayan, MD, Division of Infectious Diseases, Arkansas Children's Hospital, 1 Children's Way, Slot 512-11, Little Rock, AR 72202; vvijayan@uams.edu.

A recent outbreak in Minnesota underscores the need to maintain vigilance and adhere to best practices in immunization and containment of known...
