AGI, α-glucosidase inhibitor; DPP-4 I, dipeptidyl peptidase-4 inhibitor; GLP-1 A, glucagon-like peptide-1 agonist.
a Adapted to include sitagliptin and saxagliptin.
b Adapted to include exenatide and liraglutide.
Selecting initial therapy
Metformin, in combination with lifestyle intervention, has become the recommended first-line pharmacologic agent for the treatment of type 2 diabetes mellitus (T2DM), unless there is a contraindication, such as renal disease, hepatic disease, heart failure, gastrointestinal intolerance, or risk of lactic acidosis. This is because of its efficacy, safety, absence of weight gain, and relatively low cost.1,4 But how should therapy be modified if metformin is no longer effective in maintaining glycemic control or if it is contraindicated? Let’s return to our 3 cases and focus on the glycemic effects of the glucose-lowering agents. (The next article focuses on nonglycemic effects.) As a reminder, in these cases we address dual oral therapy failure, intolerance to metformin monotherapy, and metformin failure.
Modifying therapy
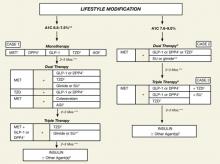
As we focus our attention on the GLP-1 agonists (exenatide, liraglutide) and DPP-4 inhibitors (sitagliptin, saxagliptin), we will keep in mind the current indications and limitations of use, as approved by the US Food and Drug Administration (FDA) (TABLE 1).15-19 We also need to keep in mind the increasing role of the GLP-1 agonists and DPP-4 inhibitors in the treatment of patients with T2DM, as reflected in the ADA/EASD 2009 consensus guidelines1 and especially the 2009 consensus statement by the AACE/ACE (FIGURE 2).4 Finally, as described in the 2010 Standards of Medical Care in Diabetes by the ADA, in reflection of recent clinical trials such as ACCORD, ADVANCE, and VADT, the target glycemic goal must be carefully determined based on many factors, including patient comorbidity, duration of T2DM, life expectancy, and history of hypoglycemia.20
TABLE 1
Approved indications and limitations of the GLP-1 agonists and DPP-4 inhibitors15-19
| Class/agents | Indications and usage | Limitations of use |
|---|---|---|
| GLP-1 agonists | ||
| Exenatide | Adjunct to diet and exercise to improve glycemic control in adults with T2DM. | Not for treatment of T1DM or diabetic acidosis. Has not been studied in combination with insulin. Has not been studied sufficiently in patients with a history of pancreatitis. Use with caution. Do not use if CrCl <30 mL/min or in ESRD. Use with caution in patients with renal transplantation. |
| Liraglutide | Adjunct to diet and exercise to improve glycemic control in adults with T2DM. | Not for treatment of T1DM or diabetic acidosis. Has not been studied in combination with insulin. Has not been studied sufficiently in patients with a history of pancreatitis. Use with caution. Not recommended as first-line therapy for patients inadequately controlled with diet and exercise. No dosing adjustment; use with caution in patients with renal disease. |
| DPP-4 inhibitors | ||
| Sitagliptin | Adjunct to diet and exercise to improve glycemic control in adults with T2DM. | Not for treatment of T1DM or diabetic acidosis. Has not been studied sufficiently in patients with a history of pancreatitis. Use with caution. Dose is reduced to 50 mg OD when CrCl ≥30 to <50 mL/min and to 25 mg OD when CrCL <30 mL/min or in ESRD requiring dialysis. |
| Saxagliptin | Adjunct to diet and exercise to improve glycemic control in adults with T2DM. | Not for treatment of T1DM or diabetic acidosis. Has not been studied in combination with insulin. Dose is reduced to 2.5 mg OD when CrCl ≤50 mL/min or in ESRD requiring dialysis. |
| DPP, dipeptidyl peptidase; ESRD, end stage renal disease; GLP, glucagon-like peptide; OD, once daily; T1DM, type 1 diabetes mellitus; T2DM, type 2 diabetes mellitus. | ||
FIGURE 2 An algorithm for glycemic control by the AACE/ACE*4,5
A1C, glycosylated hemoglobin; AGI, α-glucosidase inhibitor; DPP4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1; MET, metformin; SU, sulfonylurea; TZD, thiazolidinedione.
*To achieve A1C goal ≤6.5%, which may not be appropriate for all patients.
**For patients with diabetes and A1C <6.5%, pharmacologic Rx may be considered.
***If A1C goal not achieved safely.
†Preferred initial agent.
1DPP-4 if ↑PPG and ↑FPG or GLP-1 if ↑↑PPG.
2TZD if metabolic syndrome and/or nonalcoholic fatty liver disease (NAFLD).
3AGI if ↑PPG.
4Glinide if ↑PPG or SU if ↑FPG.
5Low-dose secretagogue recommended.
6a) Discontinue insulin secretagogue with multidose insulin; b) Can use pramlintide with prandial insulin.
7Decrease secretagogue by 50% when added to GLP-1 or DPP-4.
8If A1C <8.5%, combination Rx with agents that cause hypoglycemia should be used with caution.
9If A1C >8.5%, in patients on dual therapy, insulin should be considered.
Rodbard HW, Jellinger PS, Davidson JA, et al. Statement by an American Association of Clinical Endocrinologists/American College of Endocrinology consensus panel on type 2 diabetes mellitus: an algorithm for glycemic control. Endocr Pract. 2009;15:540-559. [Published correction appears in Endocr Pract. 2009;15:768-770]. Reprinted with permission from the American Association of Clinical Endocrinologists.
Case 1
This 53-year-old man was recently diagnosed with T2DM, with a baseline A1C of 7.5%. He is intolerant of metformin and wants to be treated with an alternative medication. In the case of metformin intolerance or a contraindication to metformin, a thiazolidinedione, DPP-4 inhibitor, GLP-1 agonist, or α-glucosidase inhibitor is recommended by the AACE/ACE if the patient’s A1C level is 6.5% to 7.5% (FIGURE 2).4,5 The safety and tolerability associated with each treatment option, such as hypoglycemia and weight gain, should be discussed with the patient.